 Short Answer Type
Short Answer TypeWhy is XeF2 linear in shape?
There are five electron pair around the xenon (two is bonding pairs and three lone pair)The arrangement of molecule is Trigonal bipyramidal. The shape is linear beacuse lone pair prefer equatorial position.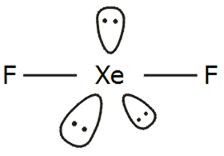
Give reason for the following:
Among the noble gases only xenon is well known to form chemical compounds.
Write chemical reaction to show that chlorine gas can be obtained from bleaching powder.
Give chemical evidence for the following:
Fluorine is a stronger oxidising agent than chlorine.
Draw the structure of P4O10 and identify the number of single and double P — O bonds.
