 Short Answer Type
Short Answer TypeAccount for the following:
For protecting electrical instruments, neon is generally used in safety devices.
Nitrogen is small in size and have ability to form multiple bonding with oxygen.thus oxides of nitrogen have open chain structures. For example N2O5.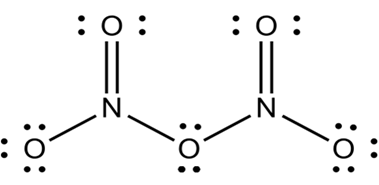
On other hand phosphorus due to its larger size does not form this type of multiple bonds with oxygen but instead forms single bonds and forms oxides with cage like structure.
For example P4O10.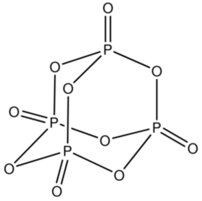
How would you account for the following:
Hydrogen fluorine is much less volatile than hydrogen chloride.
