 Multiple Choice Questions
Multiple Choice QuestionsOn heating, chloric acid decomposes to
HClO4, Cl2, O2 and H2O
HClO2, Cl2, O2 and H2O
HClO, Cl2O and H2O2
HCl, HClO, Cl2O and H2O
Which one of the following is not true at room temperature and pressure?
P4O10 is white solid
SO2 is a colourless gas
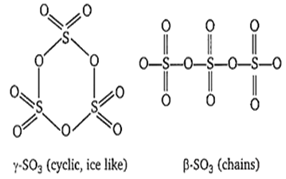
SO3 is a colourless gas
NO2 is a brown gas
C.
SO3 is a colourless gas
At room temperature SO3 is a solid and exists as either α-SO3, β-SO3 or γ-SO3. α-SO3 have similar chains cross-linked into sheets.
An element belongs to group 15 and third period of the Periodic Table. Its electronic configuration wil be
1s22s22p3
1s22s22p4
1s22s22p63s23p3
1s22s22p63s23p2
In the brown ring complex [Fe(H2O)5(NO)]SO4, nitric oxide behaves as
NO+
neutral NO molecule
NO-
NO2-
Platinum, palladium and iridium are called noble metals because
Alfred Nobel discovered them
they are shining lustrous and pleasing to look at
they are found in native state
they are inert towards many common reagents
A solution of KMnO4 is reduced to a various products depending upon its pH. At pH<7, it is reduced to a colourless solution (A), at pH =7 it forms a brown precipitate (B) and at pH> 7, it gives a green solution (C).(A), (B) and (C) are
| A | B | C |
| Mn2+ | MnO2 |
| A | B | C |
| MnO2 | Mn2+ |
| A | B | C |
| Mn2+ | MnO2 |
| A | B | C |
| Mn2+ | MnO2 |
Which two sets of reactants best represents the amphoteric character of Zn(OH)2?
Set I - Zn(OH)2(s) and OH- (aq)
Set II - Zn(OH)2(s) and H2O (I)
Set III - Zn(OH)2 (s) and H+ (aq)
Set IV - Zn(OH)2(s) and NH3 (aq)
III and II
I and III
IV and I
II and IV
