 Multiple Choice Questions
Multiple Choice QuestionsAn organic compound having carbon, hydrogen and sulphur contains 4% of sulphur. The minimum molecular weight of
the compound is
200
400
600
800
Amongest NO, AsO-, CO, ClO, SO and BO, the non-planar species are
CO
AsO
NO
SO
Which of the following hydrogen bonds are strongest in vapour phase?
HF..........HF
HF..........HCl
HCl..........HCl
HF..........HI
Compound A and B are treated with dil. HCl separately. The gases liberated are Y and Z respectively. Y turns acidified dichromate paper green while Z turns lead acetate paper black. The compound A and B are respectively.
Na2CO3 and NaCl
Na2SO3 and Na2S
Na2S
Na2SO4
Fluorine reacts with dilute NaOH and forms a gaseous. product A. The bond angle in the molecule of A is
104°40'
103°
107°
109°28'
B.
103°
![]()
The structure of 'A' (OF2) is as
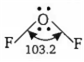
![]() bonds made by O = 2
bonds made by O = 2
Lone pairs of electrons on O = 2
No. of orbita. used by O for hybridisation
=2+2=4
Hybridisation of O in OF2 = sp3
Due to repulsion between two lone pairs of electrons, its shape gets distorted. Therefore, the bond angle in the molecule is 103°.
The number of
3,4
4,2
2,3
3,2
The type of bonds present in sulphuric anhydride are
