 Multiple Choice Questions
Multiple Choice QuestionsWhich pair of oxyacids of phosphorus contains 'P - H' bonds?
H3PO4, H3PO3
H3PO5, H4P2O7
H3PO3, H3PO2
H3PO2, HPO3
AgCl dissolves in a solution of NH3 but not in water because :
NH3 is a better solvent than H2O
Ag+ forms a complex ion with NH3
NH3 is a stronger base than H2O
the dipole moment of water is higher than NH3
What is the product formed when acetylene reacts with hypochlorous acid ?
CH3COCl
ClCH2CHO
Cl2CHCHO
ClCH2COOH
As the number of -OH groups increases in hypophosphorus acid, phosphorus acid and phosphoric acid, the acidic strength
increases
decreases
remains nearly same
remains appropriately same
C.
remains nearly same
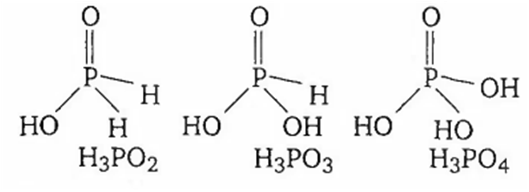
Although the number of -OH groups is increasing in H3PO2 (1 OH group), H3PO3 (2 OH group) and H3PO4 (3 OH group), yet acidity does not increase much. This is due to the fact that the number of unprotonated oxygen, responsible for enhancement of acidity due to inductive effect, remains the same, as a result dissociation constant also remain nearly same.
The comparatively high b.pt. of HF is due to
high reactivity of fluorine
small size of hydrogen atom
formation of hydrogen bonds and consequent association
high IE of fluorine
