 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following property does not correspond to the order HI < HBr < HCl < HF ?
Thermal stability
Reducing power
Ionic character
Dipole moment
Which of the following phosphorus oxyacids can act as a reducing agent?
H3PO3
H3PO4
H2P2O6
H4P2O7
Argon possesses
translational motion only
translational + rotational motion
translational + vibrational motion
translational + rotational + vibrational motion
Which among the following group 16 elements exists in more than two allotropic states?
Polonium
Tellurium
Selenium
Oxygen
Electronic configuration of only one p-block element is exceptional. One molecule of that element consists of how many atoms of it?
One
Two
Three
Four
Which oxyacid of sulphur contains S-S single bond?
Oleum
Marshall's acid
Dithionic acid
Thiosulphuric acid
Which among the following group 15 element forms most stable pentavalent compound?
Phosphorus
Antimony
Bismuth
Arsenic
What is the basicity of orthophosphorous acid?
One
Two
Three
Four
B.
Two
The structure of ortho phosphorous acid will be
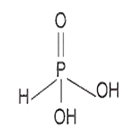
Here, two -OH groups are directly attached with P-atom, hence the basicity of the compound is two.
Which oxoacid of sulphur contains S-S bond in its structure?
Disulphurous acid
Disulphuric acid
Perdisulphuric acid
Hydrosulphurous acid
