 Multiple Choice Questions
Multiple Choice QuestionsWhich halogen forms an oxyacid that contains the halogen atom in tripositive oxidation state?
Fluorine
Chlorine
Bromine
Iodine
Identify the compound, in which phosphorus exists in the oxidation state of +1.
Phosphonic acid (H3PO3)
Phosphinic acid (H3PO2)
Pyrophosphorus acid (H4P2O5)
Orthophosphoric acid (H3PO4)
In preparation of sulphuric acid from sulphur dioxide in lead chamber process. What substance is used as a catalyst?
Manganese dioxide
Vanadium pentoxide
Nitric oxide
Raney nickel
Which halogen has the highest value of negative electron gain enthalpy?
Fluorine
Chlorine
Bromine
Iodine
Freon 12 is manufactured from CCl4 by
Wurtz reaction
Swarts reaction
Fittig reaction
Wurtz-Fittig reaction
In which one of the following compounds of xenon, highest number of lone pair of electrons is present in xenon?
XeF6
XeF4
XeF2
XeOF3
Which one of the following has the maximum number of P-OH bonds?
H3PO2
H3PO4
H4P2O6
H4P2O5
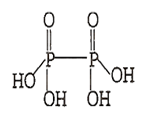
C.
H4P2O6
H4P2O6 has 4 P-OH bonds.
Which one of the following statements is not true in respect of propeties of interhalogen compounds?
They are all covalent compounds
They are volatile solids or liquids except ClF
IF5 has square pyramidal structure
They are all paramagnetic in nature
Which of the following noble gases has the nighest positive electron gain enthalpy value?
Helium
Krypton
Argon
Neon
