 Multiple Choice Questions
Multiple Choice QuestionsWhich one is the correct order of acidity?
CH2= CH2> CH3– CH = CH2> CH3– C ≡ CH > CH ≡ CH
CH ≡ CH > CH3– C ≡ CH > CH2= CH2> CH3– CH3
CH ≡ CH > CH2= CH2> CH3– C ≡ CH >CH3– CH3
CH ≡ CH > CH2= CH2> CH3– C ≡ CH >CH3– CH3
The heating of phenyl-methyl ethers with HI produces.
Ethyl chlorides
Iodobenzene
Phenol
Phenol
The compound A on treatment with Na gives B, and with PCl5 gives C. B and C react together to give diethyl ether. A, B and C are in the order
C2H5OH, C2H6, C2H5Cl
C2H5OH, C2H5Cl, C2H5ONa
C2H5OH, C2H5ONa, C2H5Cl
C2H5Cl, C2H6, C2H5OH
In the reaction,
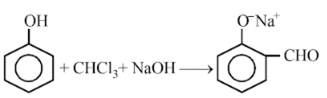
The electrophile involved is
Dichloromethyl cation (C+HCl2)
Formyl cation (C+HO)
Dichlorocarbene (:CCl2)
Dichloromethyl anion (C-HCl2)
Aqueous 10% NaHCO3 solution is used as a reagent for identifying 'A'. Which of the following compounds yield 'A' on hydrolysis?
CH3COOC2H5
C2H5-COO-C2H5
CH3CHO
CH3CH2OH
When a mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous NaHCO3 solution, then which of the following is right distribution?
| In ether | In sodium bicarbonate solution |
| Sodium hexanoate | 1- hexanol |
| 1- hexanol | Hexanoic acid |
| 1- hexanol | Sodium hexanoate |
| Hexanoic acid | 1- hexanol |
The correct order of increasing acidic strength is
phenol < ethanol < chloroacetic acid < acetic acid
ethanol < phenol < chloroacetic acid < acetic acid
ethanol < phenol < acetic acid < chloroacetic acid
chloroacetic acid < acetic acid < phenol < ethanol
