 Multiple Choice Questions
Multiple Choice QuestionsThe alkene that exhibits geometrical isomerism is
propene
2-methyl propene
2-butene
2-butene
Which of the following has an optical isomer ?
[CO(en)(NH3)2]2+
[CO(H2O)(en)]3+
[CO(H2O)(en)]3+
The number of stereoisomers possible for a compound of the molecular formula CH3-CH =CH-(OH)-Me is
3
2
4
4
Which of the following pairs represents linkage isomers?
[Cu(NH3)4][PtCl4] and [Pt(NH3)4][CuCl4]
[Pd(PPh3)2(NCS)2] and [Pd(PPh3)2(SCN)2]
[CO(NH3)5NO3]SO4 and [CO(NH3)5SO4]NO3
[CO(NH3)5NO3]SO4 and [CO(NH3)5SO4]NO3
The coordination number and the oxidation state of the element ‘E’ in the complex [E(en)2(C2O4)]NO2 (where(en) is ethylene diamine) are, respectively,
6 and 2
4 and 2
4 and 3
4 and 3
D.
4 and 3
In which of the following octahedral complexes of Co (at. no. 27), will the magnitude of ∆o be the highest?
[Co(CN)6]3‚àí
[Co(C2O4)3]3‚àí
[Co(H2O)6]3+
[Co(H2O)6]3+
Which one of the following has a square planar geometry?
[CoCl4]2-
[FeCl4]2–
[NiCl4] 2–
[NiCl4] 2–
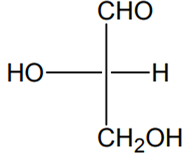
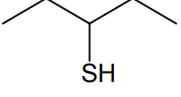
Which of the following molecules is expected to rotate the plane of plane-polarised light?


In which of the following ionization processes, the bond order has increased and the magnetic behaviour has changed?
C2 ‚Üí C2+
NO ‚Üí NO+
O2 ‚Üí O2+
O2 ‚Üí O2+
