 Multiple Choice Questions
Multiple Choice QuestionsFor a spontaneous reaction the ∆G, equilibrium constant (K) and Eocell will be respectively
-ve, >1, +ve
+ve, >1, -ve
-ve, <1, -ve
-ve, <1, -ve
The exothermic formation of ClF3 is represented by the equation
Which of the following will increase the quantity of ClF3 in an equilibrium mixture of Cl2, F2 and ClF3?
Increasing the temperature
Removing Cl2
Increasing the volume of the container
Increasing the volume of the container
For the reaction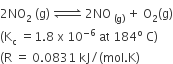
When Kp and Kc are compared at 184oC, it is found that
Kp is greater than Kc
Kp is less than Kc
Kp = Kc
Kp = Kc
Hydrogen ion concentration in mol / L in a solution of pH = 5.4 will be
3.98 x 108
3.88 x 106
3.68 x 10-6
3.68 x 10-6
Among the following acids which has the lowest pKa value?
CH3COOH
HCOOH
(CH3)2COOH
(CH3)2COOH
What is the equilibrium expression for the reaction P4(s) +5O2(g)⇌ P4O10(s)?
Kc = [P4O10] / P4] [O2]5
Kc = 1/[O2]5
Kc = [O2] 5
Kc = [O2] 5
The equilibrium constant for the reaction N2(g) + O2(g) ⇌ 2NO(g) at temperature T is 4×10-4. The value of Kc for the reaction NO(g) ⇌ 1/2N2(g) + 1/2O2(g) at the same temperature.
2.5×102
0.02
4 x 10-4
4 x 10-4
