 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following reacts with benzene in presence of anhydrous aluminum chloride and forms acetophenone ?
CH3Cl
CH3COOH
CH3CHO
CH3COCl
Which of the following reactions does not liberate gaseous product ?
AlCl3 + NaOH →
NaOH + P(white) + H2O →
Al + NaOH
Zn + NaOH
CH3CH2OH CH3CHO Cl3CCHO
In the above reaction, the role of Cl2 in Step-1 and Step-2 respectively is:
oxidation, chlorination
reduction, chlorination
oxidation, addition
reduction,substitution
[Co(NH3)5SO4]Br and [Co(NH3)5Br]SO4 are a pair of ....... isomers.
ionisation
ligand
coordination
hydrate
According to Cahn-Ingold-Prelog sequence rules, the correct order of priority for the given groups is
-COOH > -CH2OH > -OH > -CHO
-COOH > -CHO > -CH2OH > -OH
-OH > -CH2OH > -CHO > -COOH
-OH > -COOH > -CHO > -CH2OH
What are X and Y respectively in the following reaction?
Z-product 2-butyne E-product
Na/ NH3 (liq) and Pd/ BaSO4 + H2
Ni/ 140°C and Pd/ BaSO4 + H2
Ni/ 140°C and Na/ NH3 (liq)
Pd/ BaSO4 + H2 and Na/ NH3 (liq)
The IUPAC name of the following compound is-
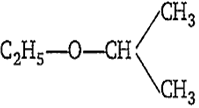
ethoxy propane
1,1-dimethyl ether
2-ethoxy isopropane
2-ethoxy propane
Calorific value of producer gas is low because of
high percent of N2
low percent of CO2
high percent of CO
low percent of N2
One mole of alkene on ozonolysis gave one mole of acetaldehyde and one mole of acetone. The IUPAC name of is
2-methyl-2-butene
2-methyl-1-butene
2-butene
1-butene
The concentration of an organic compound in chloroform is 6.15 g per 100 mL of solution. A portion of this solution in a 5 cm polarimeter tube causes an observed rotation of -1.2°. What is the specific rotation of the compound?
+ 12°
-3.9°
-39°
+61.5°
