 Multiple Choice Questions
Multiple Choice QuestionsDuma's method involves the determination of nitrogen content in the organic compound in the form of
NH3
N2
NaCN
(NH4)2SO4
In Duma's method of estimation of nitrogen, 0.35 g of an organic compound gave 55 mL of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be (Aqueous tension at 300 K = 15 mm)
16.45
17.45
14.45
15.45
Which of the following is not correct?
Ammonia is used as refrigerant
A mixture of Ca(CN)2 and C is known as nitrolim
A mixture of Ca(H2PO4),% and CaSO4.2H20 is known as superphosphate of lime
Hydrolysis of NCl3 gives NH3 and HOCl
Which one of the following is aromatic?
Cyclopentadienyl cation
Cyclooctatetraene
Cycloheptatriene
Cycloheptatrienylcation
The complexes [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6] are the examples of which type of isomerism?
Ionisation isomerism
Coordination isomerism
Geometrical isomerism
Linkage isomerism
Which of the following compounds is soluble in benzene but almost insoluble in water?
C2H5OH
CH3CO2H
CH3CHO
C6H5NO2
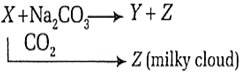
The chemical formulae of X, Y and Z are
| X | Y | Z |
| CaO | Ca(OH)2 | NaOH |
| NaOH | CaO | CaCO3 |
| NaOH | Ca(OH)2 | CaCO3 |
| Ca(OH)2 | NaOH | CaCO3 |
What is correct about the following structure?
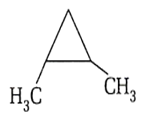
Total stereoisomers = 4
Number of chiral carbons = 1
Number of optical isomers= 2
Number of meso compounds = 2
During estimation ofnitrogen in the organic compound by Kjeldahl's method, the ammonia evolved from 0.5 g of the compound in Kjeldahl's estimation of nitrogen, neutralised 10 mL of 1M H2SO4. Find out the percentage of nitrogen in the compound.
14%
28%
56%
68%
