 Multiple Choice Questions
Multiple Choice QuestionsThe correct order of stability of carbocations, is :
1 < 2 < 3 < CH3
H3 < 1 < 2 < 3
3 < 2 < 1 < H3
2 < 3 < 1 < H3
The IUPAC name of the following compound is
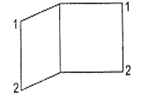
bicyclo [2, 2, 0] octane
bicyclo [0, 2, 2] hexane
bicyclo [2, 1, 1] hexane
bicyclo [2, 2, 0] hexane
The following compound can not exhibit
CH3CH = CH - CH(OH).COOH
geometrical isomerism
optical isomerism
metamerism
position isomerism
Which of the following statement is not true about homolytic cleavage?
In homolytic cleavage the movement of a single electron takes place instead of an electron pair.
The organic reaction which proceed through homolytic bond cleavage are called ionic reactions or polar reactions
Homolytic cleavage results in the formation of neutral species which contains an unpaired electrons
None of the above
Which of the following has the maximum acidic strength ?
p-nitrophenol
p-nitrobenzoic acid
o-nitrobenzoic acid
m-nitrobenzoic acid
A light greenish coloured salt was soluble in water. On passing H2S into the solution a black precipitate was obtained which dissolved readily in HCl The metal ion present is:
Co2+
Fe2+
Ni2+
Mn2+
What is 'X' in the following sequence ?
ArN2Cl Z Y X
Benzoic acid
Sodium benzoate
Benzaldehyde
Benzene
A salt on reacting with dil. H2S04 gives a reddish brown gas. The salt is:
KNO2
ZnBr2
NaNO3
FeCl3
