 Multiple Choice Questions
Multiple Choice QuestionsEase of nucleophilic addition in the given compounds is
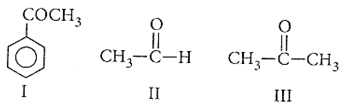
I > III > II
II > III > I
II > I > III
III > I > II
Which of the following species is not aromatic?
Benzene
Cyclooctatetraenyl dianion
Tropylium ion
Cyclopentadienyl cation
Select the correct statement.
Geometrical isomer may differ in dipole moment and visible/UV spectra.
Complexes of the type [Ma3b3] can also have facial (fac) and meridional (mer) isomer.
No optical isomer exists for the complex trans- [Co(en)2Cl2]+.
All of these.
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution :
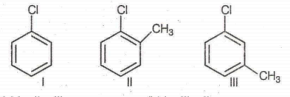
I < II < III
I < III < II
III < II < I
II < III < I
Among the following, the achiral amino acid is
2-ethylalanine
2-methylglycine
2-hydroxymethylserine
tryptophan
Assertion : When acetamide reacts with NaOH and Br,, methyl amine is formed.
Reason : The reaction occurs through intermediate formation of isocyanate.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false
If both assertion and reason are false
Assertion : Chlorobenzene is more reactive than benzene towards the electrophilic substitution reaction.
Reason : Resonance destabilises the carbocation.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false
If both assertion and reason are false
Assertion : The Dumas method is more applicable to nitrogen containing organic compounds than the Kjeldahl's method.
Reason: The Kjeldahl's method does not give satisfactory results for compounds in which nitrogen is directly linked to oxygen.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false
If both assertion and reason are false
Isopropylbenzene on air oxidation in the presence of dilute acid gives
C6H5COOH
C6H5COCH3
C6H5CHO
C6H5OH
