 Multiple Choice Questions
Multiple Choice QuestionsOptical isomerism is exhibited by (ox= oxalate anion; en= ethylenediamine).
cis-[CrCl2(ox)2]3-
[Co(en)3]3+
trans-[CrCl2(ox)2]3-
[Co(ox) (en)2]+
The IUPAC name of the following molecule is
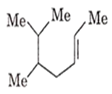
5,6-dimethylhept-2-ene
2,3-dimethylhept-5-ene
5,6-dimethylhept-3-ene
5-iso-propylhex-2-ene
The reagents to carry out the following conversion are
![]()
HgSO4/dil. H2SO4
BH3; H2O2/NaOH
OSO4; HIO4
NaNH2/ CH3I; HgSO4/ dil.H2SO4
The well-known compounds, (+)- lactic acid and (-)- lactic acid, have the same molecular formula, C3H6O3. The correct relationship between them is:
constitutional isomerism
geometrical isomerism
identicalness
optical isomerism
Which one of the following characteristics belong to an electrophile?
It is any species having electron deficiency which reacts at an electron-rich C-centre
It is any species having electron enrichment, that reacts at an electron-deficient C-centre
It is cationic in nature
It is anionic in nature
Among the following structures the one which is not a resonating structure of others is
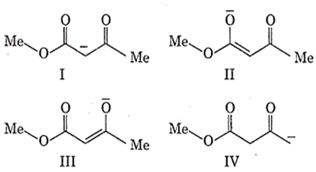
I
II
III
IV
Among the following carbocations:
Ph2C+CH2Me (I), PhCH2CH2CH+Ph (II), Ph2CHCH+Me (III) and Ph2C(Me)CH2 (IV), the order of stability is:
IV > II > I > III
I > II > III > IV
II > I > IV > III
I > IV > III > II
Which one of the following will show optical isomerism?
OH-CH2-CO2H
CH3-CH(OH)-CO2H
(CH3)2-CH-CO2H
(CH3)2-C(Cl)-CO2H
Among the following statements about the molecules X and Y, the one(s) which correct is (are)
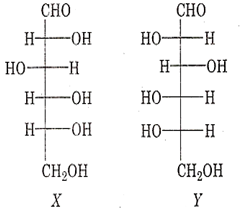
X and Y are diastereomers
X and Y are enantiomers
X and Y are both aldohexoses
X is a D-sugar and Y is an L-sugar
 Short Answer Type
Short Answer Type