 Multiple Choice Questions
Multiple Choice QuestionsAn aqueous dilute solution containing non-volatile solute boils at 100.52° C. What is the molality of solution?(Kb = 0.52 kg mol-1K, boiloing temperature of water = 100° C)
0.1 m
0.01 m
0.001 m
1.0 m
The experimental depression in freezing point of a dilute solution is 0.025 K. If the van't Hoff factor (i) is 2.0, the calculated depression in freezing point (in K) is
0.00125
0.025
0.0125
0.05
The molality of an aqueous dilute solution containing non-volatile solute is 0.1 m. What is the boiling temperature (in °C) of solution? (Boiling point elevation constant, Kb = 0.52 kg mol-1K; boiling temperature of water = 100°C).
100.0052
100.052
100.0
100.52
When helium gas is allowed to expand into vaccum, heating effect is observed. The reason for this is (assume He as a non ideal gas)
He is an inert gas
The inversions temperature of helium is very high
The inversion temperature of helium is very low
He has the lowest boiling point
Which of the following conditions are correct for real solutions showing negative deviation from Raoult's law?
ΔHMix <0, ΔVMix > 0
ΔHMix >0, ΔVMix > 0
ΔHMix >0, ΔVMix < 0
ΔHMix <0, ΔVMix <0
Molal depression constant for a solvent is 4.0 K kg mol-1. The depression in the freezing point of the solvent for 0.03 mol kg-1 solution of K2SO4 is : (Assume complete dissociation of the electrolyte)
0.24 K
0.12 K
0.18 K
0.36 K
For the solution of the gases w, x, y and z in water at 298 K, the Henrys law constants (KH) are 0.5, 2, 35 and 40 kbar, respectively. The correct plot for the given data is :
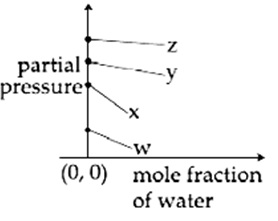
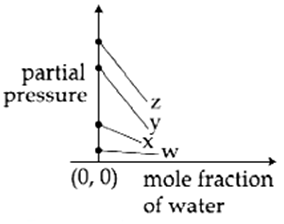
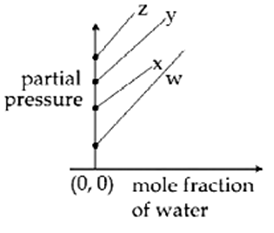
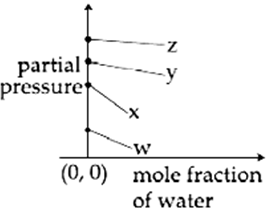
The osmotic pressure of a dilute solution of an ionic compound XY in water is four times that of a solution of 0.01 M BaCl2 in water. Assuming complete dissociation of the given ionic compounds is water, the concentration of XY (in mol L-1 ) in solution is
4 × 10-4
16 × 10-4
4 × 10-2
6 × 10-2
At room temperature a dilute solution of urea is prepared by dissolving 0.60g of urea in 360g of water . If the vapour pressure of pure water at this temperature is 35mmHg , Lowering of vapour pressure will be (molar mass of urea =60g mol-1).
0.28mmHg
0.031 mmHg
0.027Hg
0.017mmHg
