 Multiple Choice Questions
Multiple Choice QuestionsIn which process the rate of transfer of heat is maximum?
Conduction
Radiation
Convection
In all three heat is transferred with the same speed
According to Newton's law of cooling, the rate of cooling of a body is proportional to (Δθ)n, where Δθ is the difference of the temperature of the body and the surroundings, and n is equal to
2
3
4
1
A cup of tea having the temperature 80°C is kept in a room,which is at 20°C. Which of the given curves best represents the variation of temperature T of the cup with time t ?

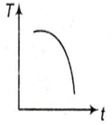


An ice box made of Styrofoam (thermal conductivity = 0.01 Jm-1s-1K) is used to keep liquids cool. It has a total wall area including lid of 0.8 m2 and wall thickness of 2.0 cm. A bottle of water is placed in the box and filled with ice. If the outside temperature is 30°C, the rate of flow of, heat into the box is (in Js-1)
16
14
12
10
5 g of ice of O°C is dropped in a beaker containing 20 g of water at 40°C. The final temperature will be
32°C
16°C
8°C
24°C
An electric heater of resistance 6 Ω works for 10 min on a 60 V line. The energy liberated in this period of time is
7.2 × 103 J
3.6 × 105 J
43.2 × 104 J
28.8 × 104 J
The amount of heat required to change 1 g (0°C) of ice into water of 100°C, is
716 cal
500 cal
180 cal
100 cal
