 Multiple Choice Questions
Multiple Choice QuestionsA diatomic ideal gas is used in a Carnot engine as the working substance. If during the adiabatic expansion part of the cycle the volume of the gas increases from V to 32 V,the efficiency of the engine is
0.5
0.75
0.99
0.99
Two points P and Q are maintained at the potential of 10V and -4V respectively. The work done in moving 100 electrons from P to Q is
9.60 × 10–17 J
9.60 × 10–17 J
– 2.24 × 10–16 J
– 2.24 × 10–16 J
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P–T diagram.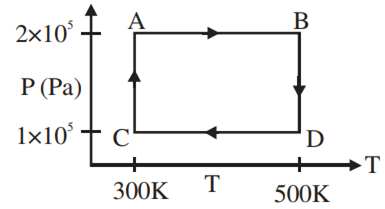
The net work done on the gas in the cycle ABCDA is
zero
276 R
1076 R
1076 R
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P–T diagram.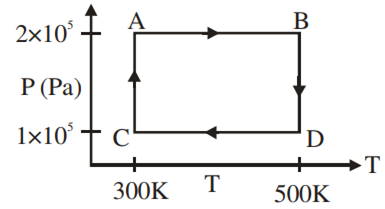
The work done on the gas in taking it from D to A is
– 414 R
414 R
-690 R
-690 R
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P–T diagram. 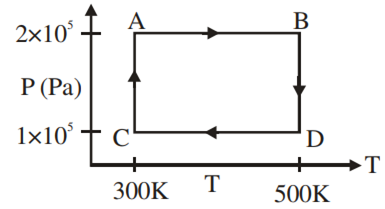
Assuming the gas to be ideal the work done on the gas in taking it from A to B is
200 R
300 R
400 R
400 R
An insulated container of gas has two chambers separated by an insulating partition. One of the chambers has volume V1 and contains ideal gas at pressure P1 and temperature T1. The other chamber has volume V2 and contains ideal gas at pressure P2 and temperature T2. If the partition is removed without doing any work on the gas, the final equilibrium temperature of the gas in the container will be
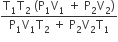
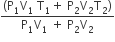
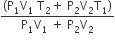
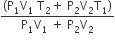
A Carnot engine, having an efficiency of η = 1/10 as heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
99 J
90 J
1 J
1 J
When a system is taken from state i to state f along the path iaf, it is found that Q = 50 cal and W = 20 cal. Along the path’ ibf Q = 36 cal. W along the path ibf is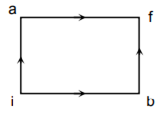
6 cal
66 cal
16 cal
16 cal
The work of 146 kJ is performed in order to compress one kilo mole of gas adiabatically and in this process the temperature of the gas increases by 7° C. The gas is
(R = 8.3 J mol−1 K−1 )
monoatomic
diatomic
triatomic
triatomic
Which of the following is incorrect regarding the first law of thermodynamics?
It introduces the concept of internal energy
It introduces the concept of entropy
It is not applicable of any cyclic process
It is not applicable of any cyclic process
