 Multiple Choice Questions
Multiple Choice QuestionsDecreasing order of reactivity in Williamson synthesis of the following :
I. Me3CCH2Br
II. CH3CH2CH2Br
III. CH2=CHCH2Cl
IV. CH3CH2CH2Cl
III > II > IV > I
I > II > IV > III
II > III > IV > I
I > III > II > IV
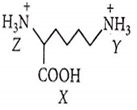
Arrange X, Y and Z in order of increasing acidic strengths.
X > Z > Y
Z < X < Y
X > Y > Z
Z > X > Y
The pKa of phenol is 10. Which of the following bases will deprotonate phenol completely?
NaNH2 (PKa of NH3 = 35) , (PKb of H2O = 16) , NaNH2 (pKa of N = 10) , NaOOCH3 (pKb of CH3COOH =5)
NaNH2
NH3
NaNH2 and NaOH
NH3 and NaOCOCH3
Assertion : Oxidation of 1-nitronaphthalene gives o-nitrophthalic acid whereas 1-amino naphthalene on oxidation gives phthalic acid.
Reason : An amino group attached to the benzene ring makes it resistant to oxidation whereas nitro group makes the benzene ring susceptible to oxidation.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion: Phenol forms 2, 4, 6-tribromophenol on treatment with Br2- water at 273 K.
Reason : Phenol is o, p- directing group.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false
If both assertion and reason are false
The acidic strength of the given compounds follows the order
I. CH3-CH=CH-COOH
II. CH3--CH=CH-COOH
III. CH3=CH2-COOH
II > III > I
III > II > I
II > I > III
I > II > III
The reaction,
C6H5ONa + CO2 +H2O C6H5OH + NaHCO3 suggests that
phenol is a stronger acid than carbonic acid
carbonic acid is a stronger acid than phenol
water is a stronger acid than phenol
None of these.
Phenol , when it first react with cone. sulphuric acid and then with cone. nitric acid gives :
nitrobenzene
2 , 4 , 6 - trinitrobenzene
o - nitrophenol
p - nitrophenol
During dehydration of alcohols to alkenes by heating with conc. H2SO4 , the initial step is :
formation of an este
protonation of alcohol molecule
formation of carbocation
elimination of water
Assertion : Phenol is more acidic than ethanol.
Reason : Phenoxide ion is resonance stabilised.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
