 Multiple Choice Questions
Multiple Choice QuestionsAssertion: Ethers behave as bases in the presence of mineral acids.
Reason: It is due to the presence of a lone pair of electrons on the oxygen.
If both assertion and reason are true and the reason is a correct explanation of the assertion.
If both the assertion and reason are true but the reason is not a correct explanation of the assertion.
If the assertion is true but the reason is false.
If both the assertion and reason are false.
Which one of the following properties are exhibited by phenol?
It is soluble in aq. NaOH and evolves CO2 with aq. NaHCO3
It is soluble in aq. NaOH and does not evolve CO2 withe aq. NaHCO3
It is not soluble in aq. NaOH but evolves CO2 with aq. NaHCO3
It is insoluble in aq. NaOH and does not evolve CO2 with aq. NaHCO3
When phenol is treated with D2SO4 /D2O, some of the hydrogens get exchanged. The final product in this exchange reaction is
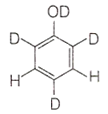
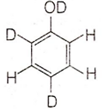
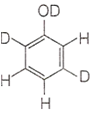
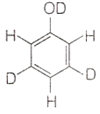
The correct order of decreasing acidity of nitrophenols will be:
m-nitrophenol > p-nitrophenol > o-nitrophenol
o-nitrophenol > m-nitrophenol > p-nitrophenol
p-nitrophenol > m-nitrophenol > o-nitrophenol
p-nitrophenol > o-nitrophenol > m-nitrophenol
Among the following ethers, which one will produce methyl alcohol on treatment with hot concentrated HI?
X C2H5Cl, Y CH3COCl
X and Y are
(C2H5)2O and CH3CO2H
C2H5I and C2H5CHO
C2H5OH and CH3CO2H
C2H5OH and C2H5CHO
 Short Answer Type
Short Answer TypeBoth phenol and alcohol contains -OH group. But still phenol is more acidic than alcohol. Explain why?
 Multiple Choice Questions
Multiple Choice QuestionsWhich statement is incorrect?
Phenol is a weak acid
Phenol is an aromatic compound
Phenol liberates CO2 from Na2CO3 solution
Phenol is soluble in NaOH
