 Multiple Choice Questions
Multiple Choice QuestionsIdentify the correct decreasing order of acidity of the given carboxylic acid.
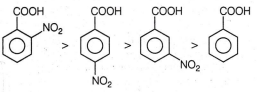
D.

-NO, group at any position shows electron withdrawing effect, thus acidic strength is increased but o-nitrobenzoate ion is stabilised by
intramolecular H-bonding hence its acidic strength is maximum.
Further, -I effect is more pronounced at p-position.
In the reaction
What are A and B?
and
CH3CH2 OMg I and C2H5-O-C2H5
CH3CH2O Mg I and CH3-CH2-OH
CH3-CH2-I and CH3-CH2-OH
In the given reaction,
CH3-CH2-CH2-O-CH2-CH3 [X]+[Y]
[X]+[Y] are respectively.
CH3-CH2-CH2OH and CH3-CH2-Cl
CH3-CH2-CH2-Cl and CH2-CH2-OH
CH3-CH2-CH2-Cl and CH2=CH2
CH3-CH3=CH2 and CH2=CH2
During reduction of carbonyl compounds by hydrazine and KOH, the first intermediate formed is:
RC≡N
RCONH2
RCH≡N
RCH=NH2
Which one of the following reactions cannot be used for the reduction of:
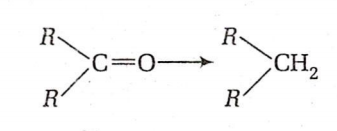
HI and red phosphorus at 200C
Wolff-Kishner reduction
Clemmensen reduction
Wurtz reaction
The compound which is not formed during the dry distillation of a mixture of calcium formate and calcium acetate is :
methanal
propanal
propanone
ethanal
