 Multiple Choice Questions
Multiple Choice Questionsd(+) lactic acid is obtained from :
fermentation of cane sugar
green vegetables
muscles
fermentation of milk sugar
Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound Eon further treatment with aqueous KOH yields compound F. Compound F is
![]()
![]()
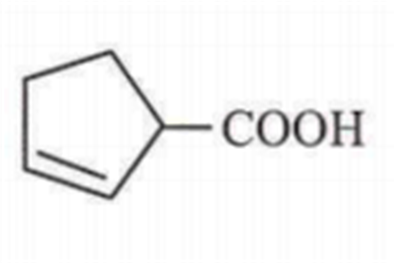
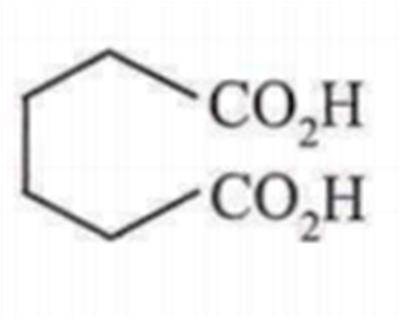
Maximum decarboxylation occurs in
CH3COOH
C6H5COOH
C6H5CH2COOH
CH3COCH2COOH
D.
CH3COCH2COOH
CH3COCH2COOH is a -keto acid. Thus, decarboxylation is maximum in a carboxylic acid containing an electron withdrawing group such as -CO or -COOH at the - carbon atom with respect to the -COOH group.
The correct increasing order of reactivity for the following molecules towards electrophilic aromatic substitution is
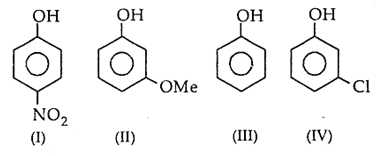
I < IV < II < III
I < IV < II < IV
I < III < II < IV
I < III < IV < II
The corrcet decreasing order of pKb is
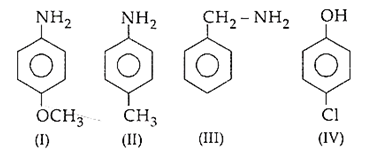
I > II > III > IV
III > IV > II > I
II > III > IV > I
IV > II > I > III
The correct decreasing order of pKa is
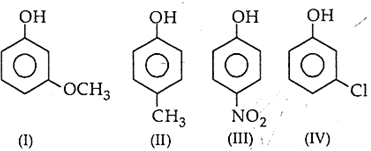
II > IV > I > III
IV > II > III > I
III > II > IV > I
IV > I > II > III
The product formed when hydroxylamine condenses with a carbonyl compound is called :
hydrazide
oxime
hydrazine
hydrazone
Asssertion: CH3-C(COOC2H5)=CH-COOH is 3-carbethoxy-2-butenoic acid.
Reason : Principal functional group gets lowest number followed by double bond or triple bond.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
