 Multiple Choice Questions
Multiple Choice QuestionsRate of a reaction can be expressed by Arrhenius equation as K= Ae-E/RT In this equation, E represents
the energy above which all the colliding molecules will react
the energy below which colliding molecules will not react
the total energy of the reacting molecules at a temperature, T
the total energy of the reacting molecules at a temperature, T
The following mechanism has been proposed for the reaction of NO with Br2 to form NOBr
NO(g) Br2 (g) ⇌ NOBr2 (g)
NOBr2 (g) NO(g)→ 2NOBr(g)
If the second step is the rate determining step, the order of the reaction with respect to NO(g) is
1
0
3
3
Hydrogen bomb is based on the principle of
Nuclear fission
Natural radioactivity
Nuclear fusion
Nuclear fusion
Consider an endothermic reaction, X → Y with the activation energies Eb and Ef for the backward and forward reactions, respectively. In general
Eb < Ef
Eb > EfEb > Ef
Eb = Ef
Eb = Ef
A reaction involving two different reactants can never be
Unimolecular reaction
First order reaction
second order reaction
second order reaction
A schematic plot of In Keq versus inverse of temperature for a reaction is shown below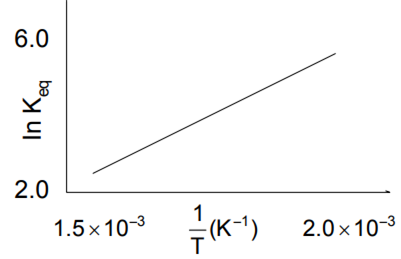
The reaction must be
exothermic
endothermic
one with negligible enthalpy change temperature
one with negligible enthalpy change temperature
The photon of hard gamma radiation knocks a proton out of 2412Mg nucleus to form
the isotope of parent nucleus
the isobar of parent nucleus
the nuclide 
the nuclide 
t1/4 can be taken as the time taken for the concentration of a reactant to drop to 3/4 of its initial value. If the rate constant for a first order reaction is K, the t1/4 can be written as
0.10 / K
0.29 / K
0.69 / K
0.69 / K
An amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0.50 atm. Pressure. Ammonium hydrogen sulphide decomposes to yield NH3 and H2S gases in the flask. When the decomposition reaction reaches equilibrium, the total pressure in the flask rises to 0.84 atm. The equilibrium constant for NH4HS decomposition at this temperature is
0.30
0.18
0.17
0.17
In first order reaction, the concentration of the reactant decreases from 0.8 M to 0.4 M in 15 minutes. The time taken for the concentration to change from 0.1 M to 0.025 M is
30 minutes
60 minutes
7.5 minutes
7.5 minutes
