 Multiple Choice Questions
Multiple Choice Questionswhich of the following does not show optical isomerism? (en = ethylenediamine)
[Co(en)2Cl2]+
[Co(NH3)3Cl3]0
[Co(en)Cl2(NH3)2]+
[Co(en)Cl2(NH3)2]+
which of the following complex ions is expected to absorb visible light?
(At. no.Zn = 30, Sc = 21, Ti = 22, Cr = 24)
[Sc(H2O)(NH3)3]3+
[Ti(en)2(NH3)2]4+
[Cr(NH3)6]3+
[Cr(NH3)6]3+
Out of TiF62-, CoF63-, Cu2Cl2 and NiCl42- (Z of Ti = 22, Co= 27, Cu = 29, Ni =28) the colourless species are
TiF62- and CoF63-
Cu2Cl2 and NiCl42-
TiF62- and Cu2Cl2
TiF62- and Cu2Cl2
Which of the following compound will exhibit cis-trans (geometrical) isomerism?
2-butene
Butanol
2-butyne
2-butyne
On the basis of the following Eo values; the strongest oxidising agent is
[Fe(CN)6]4- → [Fe(CN)6]3-] +e- ;
Eo = -0.35 V
Fe2+ → Fe3+ +e-; E = -0.77 V
[Fe(CN)6]4-
Fe2+
Fe3+
Fe3+
Which of the following complexes exhibits the highest paramagnetic behaviour?
Where gly = glycine, en = ethylenediamine and bpy = bipyridyl moities
(At. no. ; Ti = 22, V = 23, Fe =26, Co = 27)
[V(gly)2 (OH)2 (NH3)2]+
[Fe(en)(bpy)(NH3)2]+
[CO(OX)2(OH)2]-
[CO(OX)2(OH)2]-
In which of the following coordination entities the magnitude of Δo (CFSE in the octahedral field) will be maximum?
(Atomic number Co = 27)
[Co(H2O)6]3+
[CO(NH3)6]3+
[CO(CN)6]3-
[CO(CN)6]3-
C.
[CO(CN)6]3-
The magnitude of Δoct (the orbital splitting energy) is decided by the nature of ligand. Strong field ligand has highest Δoct .The increasing field strength is as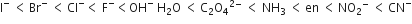
The CN- is the strongest ligand among these hence the magnitude of Δoct will be maximum in [Co(CN)6]3-
Which of the following will give a pair of enantiomorphs?
(en = NH2CH2CH2NH2)
[Co(NH3)Cl2]NO2
[Cr(NH3)6]Co(CN)6]
[Co(en)2Cl2]Cl
[Co(en)2Cl2]Cl
If there is no rotation of plane polarises light by a compound in a specific solvent, thought to by chiral, it may mean that:
the compound is certainly a chiral
the compound is certainly meso
there is no compound in the solvent
there is no compound in the solvent
