 Multiple Choice Questions
Multiple Choice QuestionsOut of the following isomeric alcohols containing five carbon atoms, the alcohol that exhibits optical isomerism is
1-pentanol
2-pentanol
3-pentanol
2-methyl-2-butanol
The IUPAC name of the complex [Co(NH3)2(H2O)4]Cl3 is
diaminetetraaquacobalt (III) trichlodde
diaminetetraaquacobalt (II) chloride
diaminetetraaquacobalt (III) chloride
tetraaquadiaminecobalt (III) trichloride
How many monochloro structural isomers are expected in free radical monochlorination of 2-methylbutane?
3
4
5
6
While assigning R, S configuration, the correct order of priority of groups attached to chiral carbon atom is
CONH2 > COCH3 > CH2OH > CHO
CONH2 > COCH3 > CHO > CH2OH
COCH3 > CH2OH > CHO > CH2OH
CHO > CH2OH > COCH3 > CONH2
The correct IUPAC name of the following compound is
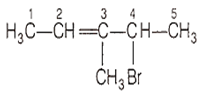
4-bromo-3-methylpent-2-ene
2-bromo-3-methylpent-4-ene
3-methyl- 4-bromopent-2-ene
3-methyl-2-bromopent-4-ene
Which one among the following cannot exhibit enantiomerism?
Diphenyl methanol
1-bromo-2-chlorobutane
2-butanol
Tartaric acid
The total number of acyclic structural isomers possible for compound with molecular formula C4H10O is
9
7
5
6
An organic compound A containing nitrogen, on acid catalysed hydrolysis produces a water soluble organic compound B and a gaseous compound C. When methyl magnesium bromide is slowly added to A in 1: 1 ratio and hydrolysed, it produces a compound which can be obtained by dry distillation of the calcium salt of B. The compound A is
N-methylmethanamide
N-ethylmethanamide
acetonitrile
N, N-dimethylmethanamide
In the estimation of sulphur by Carius method, 0.480 g of an organic compound gives 0.699 g of barium sulphate. The percentage of sulphur in this compound is (Atomic masses Ba = 137, S = 32, O = 16)
20%
15%
35%
30%
