 Multiple Choice Questions
Multiple Choice QuestionsA certain gas is taken to the five states represented by dots in the graph. The plotted lines are isotherms. Order of the most portable speed νp of the molecules at these five states is

νp at 3 > νp at 1 = νp at 2 > νp at 4 = νp at 5
νp at 1 > νp at 2 = νp at 3 > νp at 4 > νp at 5
νp at 3 > νp at 2 = νp at 4 > νp at 1 = νp at 5
insufficient information to predict the result
Two heater wires, made of the same material and having the same length and the same radius, are first connected in series and then in parallel to a constant potential difference. If the rates of I heat produced in the two cases are Hs and HP respectively, then Hs/HP will be
1/2
2
1/4
4
In an adiabatic change , the pressure and temperature of a monoatomic gas are relted as P ∝ TC, where C equals
Assertion: Ferro magnetic substances become paramagnetic above Curie temperature.
Reason: Domains are destroyed at high temperature.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
Assertion: In free expansion of an ideal gas, the entropy increases.
Reason: Entropy increases in all natural processes.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
By sucking through a straw, a student can reduce the pressure in his lungs to 750 mm of Hg ( densiy = 13.6 g/cm3). Using the straw, he can drink water from a glass upto a maximum depth of
10 cm
75 cm
13.6 cm
1.36 cm
When you make ice cubes, the entropy of water
does not change
increase
decreases
may either increase or decrease depending on the process used
A bimetallic strip consists of metals X and Y. It is mounted rigidly at the base as shown. The metal X has a higher coefficient of expansion compared to that for metal Y. When the bimetallic strip is placed in a cold bath
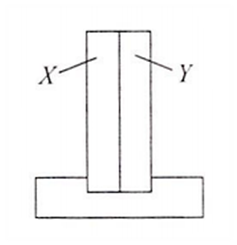
it will bend towards the right
it will bend towards the left
it will not bend but shrink
it will neither bend or shrink
Assertion: In an isolated system the entropy increases.
Reason: The processes in an isolated system are adiabatic.
If both assertion and reason are true and reason is the correct explanation of the assertion
If both assertion and reason are true but reason is not the correct explanation of the assertion
If assertion is true, but reason is false
Both assertion and reason are false statements
Assertion: The Carnot cycle is useful in understanding the performance of heat engines.
Reason: The Carnot cycle provides a way of determining the maximum possible efficiency achievable with reservoirs of given temperatures.
If both assertion and reason are true and reason is the correct explanation of the assertion
If both assertion and reason are true but reason is not the correct explanation of the assertion
If assertion is true, but reason is false
Both assertion and reason are false statements
