 Multiple Choice Questions
Multiple Choice QuestionsSalicylaldehyde can be prepared from phenol by
Scholten-Baumann reaction
Kolbe's reaction
Reimer-Tiemann reaction
Perkin reaction
Which among the following phenolic compound is most acidic in nature?
p-aminophenol
Phenol
m-nitrophenol
p-nitrophenol
Isopropylbenzene is oxidized in the presence of air to compound 'A'. When compound 'A' is treated with dilute mineral acid, the aromatic product formed is
phenol
benzene
benzaldehyde
acetophenone
Name the catalyst used in commercial method of preparation of phenol.
Silica
Calcium phosphate
Anhydrous aluminium chloride
Cobalt naphthenate
The molecular formula of Wilkinson's catalyst used in the hydrogenation of alkenes is
Co(CO)8
(Ph3P)3RhCl
[Pt(NH3)2Cl2]
K[Ag(CN)2]
Phenol can be converted to o-hydroxybenzaldehyde by
Kolbe's reaction
Reimer-Tiemann reaction
Wurtz reaction
Cannizaro reaction
When 3-phenylpropene reacts with HBr in the presence of peroxide, the major product formed is
2-bromo 1-phenylpropane
1, 2-dibromo 3-phenylpropane
3-(o-bromophenyl) propene
1-bromo 3-phenylpropane
Reaction of butanone with methylmagnesium bromide following by hydrolysis gives
2-methyl-2-butanol
2-butanol
3-methyl-2-butanol
2, 2-dimethyl-1- butanol
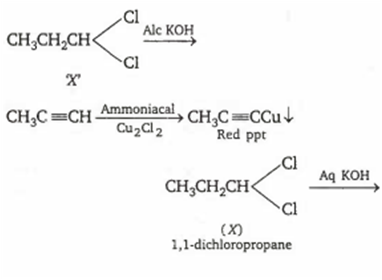
The dihalogen derivative 'X' of a hydrocarbon with three carbon atoms reacts with alcoholic KOH and produces another hydrocarbon which forms a red precipitate with ammoniacal Cu2Cl2. 'X' gives an aldehyde on reaction with aqueous KOH. The compound 'X' is
1,3-dichloropropane
1,2-dichloropropane
2,2-dichloropropane
1,1-dichloropropane
D.
1,1-dichloropropane
'X' is a three carbon compound with two halogen atom, so its molecular formula is C3H6Cl2. Only terminal alkynes give red ppt with ammoniacal Cu2Cl2 , so the hydrocarbon produced by the reaction of 'X' with ale KOH, must be a terminal alkyne (ie, CH3C≡CH)
Compound (X) gives an aldehyde when reacts with aqueous KOH. This suggests that both the halogens are present on same terminal carbon atom. Thus, the formula of compound (X) is
![]() (1,1- dichloropropane)
(1,1- dichloropropane)
and the reactions are follows:
![]()
An organic compound 'X with molecular formula, C7H8O is insoluble in aqueous NaHCO3, but dissolves in NaOH. When treated with bromine water 'X' rapidly gives 'Y, C7H5OBr3. The compounds 'X' and 'Y' respectively, are
benzyl alcohol and 2,4,6-tribromo-3-methoxy benzene
m-cresol and 2,4,6-tribromo-3-methyl phenol
benzyl alcohol and 2,4,6-tribromo-3-methyl phenol
o-cresol and 3,4,5-tribromo-2-methyl phenol
