Multiple Choice Questions
Multiple Choice QuestionsThe IUPAC name of the following compound is:
H3C--CH2-COOH
3-Hydroxy-4-methylpentanoic acid
4,4-Dimethyl-3-hydroxybutanoic acid
4-Methyl-3-hydroxypentanoic acid
2-Methyl-3-hydroxypentan-5-oic acid
In the following reaction carbonyl compound +MeOH acetal.
Rate of the reaction is highest for:
Acetone as substrate and methanol in excess
Propanal as substrate and methanol in stoichiometric amount.
Propanal as substate and methanol in excess.
Acetone as substrate and methanol in stoichiometric amount.
Major products of the following of the reaction are
![]()
![]()
CH3OH and HCO2H
![]()
![]()
A.
![]()
Aldehydes locking α – Hydrogen give cross cannizaro reaction. HCHO acts as hydride donor because it gives less sterically hindered tetrahedral Intermedia.
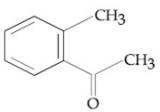
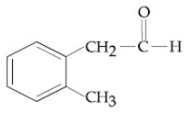
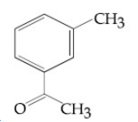
Compound A(C9H10O) shows positive iodoform rest. Oxidation of A with KMnO4/ KOH gives B(C8H6O4). Anhydride of B is used for the preparation of phenolpthalein. Compound A is:
![]()