 Multiple Choice Questions
Multiple Choice Questions100 mL of 0.01 M solution of NaOH is diluted to 1 dm3. What is the pH of the diluted solution?
12
11
2
3
Consider the following reaction equilibrium:
Initially, 1 mole of N2 and 3 moles of H2 are taken in a 2 L flask. At equilibrium state if, the number of moles of N2 is 0.6, what is the total number of moles of all gases present in the flask?
0.8
1.6
3.2
6.4
Assertion (A): The pH of a buffer solution containing equal moles of acetic acid and sodium acetate is 4.8 (pKa of acetic acid is 4.8).
Reason (R): The ionic product of water at 25°C is 10-14mol2.L-2. The correct answer is
Both (A) and (R) are true and (R) is the correct explanation of (A)
Both (A) and (R) are true and (R) is not the correct explanation of (A)
(A) is true but (R) is not true
(A) is not true but (R) is true
The equilibrium constant for the reaction
The equilibnum constant of the reaction
100 atm
200 atm
4 × 102 atm
6.25 × 104 atm
The degree of ionization of 0.10 M lactic acid is 4.0%
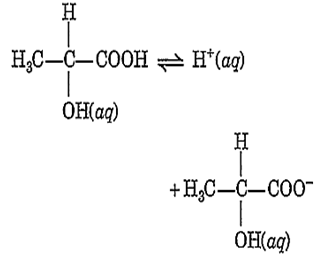
The value of Kc is
1.66 × 10-5
1.66 × 10-4
1.66 × 10-3
1.66 × 10-2
pH of a solution is 4. The hydroxide ion concentration of the solution would be
10-4
10-10
10-2
10-12
In which solution/ solvent the solubility of AgCl is minimum?
0.01 M NaCl
0.01 M CaCl2
Pure water
0.001 M AgNO3
1.0 L of 1.0 M solution of sodium hydroxide is neutralised by 1.0 L of 1.0 M of methanoic acid. If the heat of formation of water is X, the neutralisation energy of above reaction is
less than X
more than X
equal to X
None of the above
If the molar solubility of X3B3(AlF6)2 at 298 K is x, the solubility product Ksp is
18x3
27x4
27x8
2916x8
