 Multiple Choice Questions
Multiple Choice QuestionsSodium ethoxide has reacted with ethanoyl chloride. The compound that is produced in the above reaction is
Diethyl ether
2-Butanone
Ethyl chloride
Ethyl chloride
Consider the following bromides: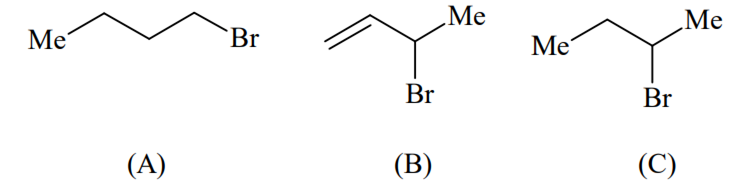
The correct order of SN1 reactivity is
A > B > C
B > C > A
B > A > C
B > A > C
The increasing order of the reactivity of the following halides for the SN1 reaction is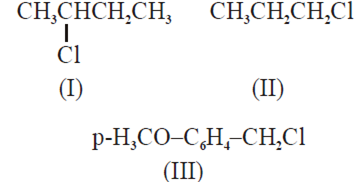
(III) < (II) < (I)
(II) < (I) < (III)
(I) < (III) < (II)
(I) < (III) < (II)
3-Methyl-pent-2-ene on reaction with HBr in presence of peroxide forms an addition product.The number of possible stereoisomers for the product is
Six
Two
Zero
Zero
The major product obtained in the following reaction is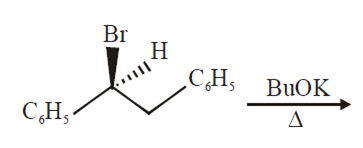
C6H5CH (OtBu) CH2C6H5
C6H5CH=CHC6H5
(+)C6H5CH(OtBu)CH2H5
(+)C6H5CH(OtBu)CH2H5
Toluene is nitrated and the resulting product is reduced with tin and hydrochloric acid. The product so obtained is diazotised and then heated with cuprous bromide. The reaction mixture so formed contains
mixture of o− and p−bromotoluenes
mixture of o− and p−dibromobenzenes
mixture of o− and p−bromoanilines
mixture of o− and p−bromoanilines
The organic chloro compound, which shows complete stereochemical inversion during a SN2 reaction,is
(C2H5)2CHCl
(CH3)3CCl
(CH3)2CHCl
(CH3)2CHCl
Which of the following reactions will yield 2, 2-dibromopropane?
CH3 – C ≡ CH + 2HBr →
CH3CH = CHBr + HBr →
CH ≡ CH + 2HBr →
CH ≡ CH + 2HBr →
