 Multiple Choice Questions
Multiple Choice QuestionsIncreasing order of reactivity of the following compounds for SN1 substitution is:
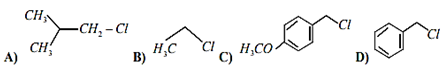
(B) < (C) < (D) < (A)
(A) < (B) < (D) < (C)
(B) < (C) < (A) < (D)
(B) < (A) < (D) < (C)
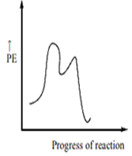
Which of the following potential energy (PE) diagrams represents the SN1 reaction?

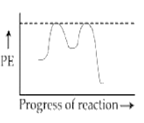
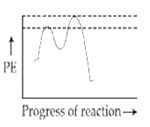
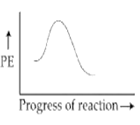
A.

For SN1 reaction, the potential energy diagram is-
The major product of the following reaction is
CH3C≡CH
CH3C (I) Cl CHD2
CH3CD (Cl) CHD (I)
CH3CD2CH(Cl) (I)
CH3CD(I)CHD (Cl)
The increasing order of reactivity of the following compounds towards aromatic electrophilic substitution reaction is
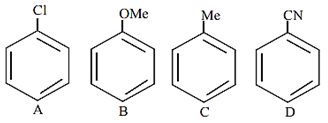
D < A < C < B
B < C < A < D
D < B < A< C
A < B < C < D
Increasing rate of SN1 reaction in the following compounds is :
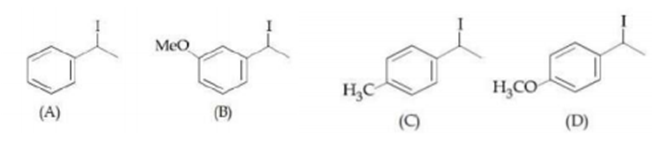
(B)<(A)<(C)<(D)
(A)<(B)<(D)<(C)
(B)<(A)<(D)<(C)
(A)<(B)<(C)<(D)
