 Multiple Choice Questions
Multiple Choice QuestionsThe maximum number of possible oxidation states of actinoides are shown by-
Neptunium (Np) and plutonium (Pu)
Nobelium (No) and lawrencium (Lr)
Berkelium (Bk) and californium (Cf)
Actinium (Ac) and throrium (Th)
The maximum possible denticities of a ligand given below towards a common transition and inner-transition metal ion, respectively, are :
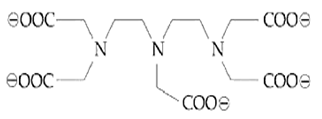
8 and 8
6 and 8
8 and 6
6 and 6
Consider the hydrated ions of Ti2+,v2+, Ti3+ and Sc3+ The correct order of their spin only magnetic moments is :
Ti3+ < Ti2+< Sc3+< V2+
Vi2+ <Ti2+< Ti3+ < Sc3+
Sc3+< Ti3+< Ti2+<V2+
Sc3+< Ti3+< V2+< Ti2+
Three complex [CoCl(NH3)5]2+ (I)[Co(NH3)5H2O]3+(II) and [Co(NH3)6]3+(III) absorb light in the visible region . the correct order of the wavelength of light absorbed by them is :
II > I > III
III > I > II
I> II> III
III> II> I
The correct order of the first ionization enthalpies is :
Ti < Mn < Ni < Zn
Mn < Ti < Zn < Ni
Ti < Mn < Zn < Ni
Zn < Ni < Mn < Ti
The highest possible oxidation states of uranium plutonium , respectively are
6 and 7
7 and 6
4 and 6
6 and 4
