How is carbon monoxide prepared on a commercial scale?

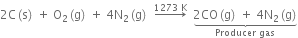
How is carbon monoxide prepared on commercial scale?
On commercial scale, it is prepared:
By the passage of steam over hot coke.
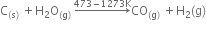
Give different uses of activated charcoal, coke.
Uses of activated charcoal.
It is used:
(i) for absorbing poisonous gas.
(ii) in filters to remove organic contaminators.
(iii) in air conditioning system to control odour.
Uses of coke.
It is used:
(i) as a fuel
(ii) as a reducing agent in metallurgy.
What is the action of heat on:
(i) Sodium bicarbonate
(ii) calcium carbonate
(iii) Zinc carbonate?
Action of heat on the given compound is,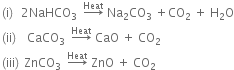
Describe what happens (give chemical equations only) when:
(i) carbon monoxide is treated with chlorine.
(ii) carbon monoxide is passed through heated NaOH under pressure.
(iii) Vapours of carbon monoxide are passed over nickel and
(iv) carbon monoxide is passed through heated ferric oxide.

