 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following pairs has the same size?
Fe2+ , Ni2+
Zr4+, Ti4+
Zr4+, Hf4+
Zr4+, Hf4+
Which one of the following ion has electronic configuration [Ar] 3d6?
(At. no: Mn = 25, Fe = 26, Co = 27, Ni = 28)
Ni3+
Mn
Fe3+
Fe3+
Among the elements Ca, Mg, P and Cl, the order of increasing atomic radii is
Mg< Ca < Cl < P
Cl < P < Mg < Ca
P < Cl < Ca < Mg
P < Cl < Ca < Mg
Among the following which one has the highest cation to anion size ratio?
CsI
CsF
LiF
LiF
Which one of the element with the following outer orbital configuration may exhibit the largest number of oxidation states?
3d5,4s2
3d5,4s1
3d5,4s2
3d5,4s2
Oxidation number of P in PO43-, of S in SO42- and that of Cr in Cr2O72- are respectively,
+5,+6 and +6
+3, + 6 and +5
+5,+3 and +6
+5,+3 and +6
Amongst the elements with following electronic configuration, which one of them may have the highest ionisation energy?
[Ne] 3s2 3p3
[Ne] 3s2 3p2
[Ar] 3d10, 4s2 4p3
[Ar] 3d10, 4s2 4p3
The correct order of decreasing second ionisation enthalpy of Ti (22), V (23), Cr(24) and Mn (25) is
Cr > Mn> V > Ti
V > Mn > Cr >Ti
Mn > Cr> Ti >V
Mn > Cr> Ti >V
The sequence of ionic mobility in aqueous solution is
K+ > Na+ > Rb+ > Cs+
Cs+ > Rb+ > K+ >Na+
Rb+ > K+> Cs+ > Na+
Rb+ > K+> Cs+ > Na+
B.
Cs+ > Rb+ > K+ >Na+
The smaller the size of ion, the greater the degree of hydration.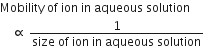
Generally in a group size or radii of ions increases, So Li has the smallest size thus bind a maximum number of water molecules with itself and becomes largest in size (in aqueous solution). Hence, the order of ionic radii (size) of alkali metal ions in aqueous solution is
Li+ > Na+ > K+> Rb+ >Cs+
Thus, the order of mobility
Which of the following oxidation states are the most characteristics for lead and tin respectively?
+4,+2
+2,+4
+4,+4
+4,+4
