 Multiple Choice Questions
Multiple Choice QuestionspH of a saturated solution of Ba(OH)2 is 12. The value of solubility product Ksp of Ba(OH)2 is
3.3 x 10-7
5.0 x 10-7
4.0 x 10-6
4.0 x 10-6
Equimolar solutions of the following substances were prepared separately, which one of these will record the highest pH value?
BaCl2
AlCl3
LiCl
LiCl
Buffer solutions have constant acidity and alkalinity because
these give unionised acid or base on reaction with added acid or alkali
acids and alkalies in these solutions are shielded from attack by other ions.
they have a large excess of H+ or OH- ions
they have a large excess of H+ or OH- ions
Give that the equilibrium constant for the reaction,
2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction t the same temperature?
SO3 (g) ⇌ SO2 (g) +1/2 O2 (g)
1.8 x 10-3
3.6 x 10-3
6.0 x 10-2
6.0 x 10-2
Given the reaction between two gases represented by A2 and B2 to give the compound AB (g).
A2(g) +B2 (g) ⇌ 2AB (g)
At equilibrium the concentration
of A2 = 3.0 x 10-3 M
of B2 = 4.2 x 10-3 M
of AB = 2.8 x 10-3 M
If the reaction takes place in a sealed vessel at 527oC, then the value of Kc will be
2.0
1.9
0.62
0.62
A buffer solution is prepared in which the concentration of NH3 is 0.30 M and the concentration of NH4 is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1.8 x 10-5, what is the pH of this solution?
log ( 2.7 = 0.43)
9.43
11.72
8.73
9.08
The value of ΔH for the reaction
X2 (g) + 4Y2 (g) ⇌ 2XY4 (g) is less than zero. Formation of XY4 (g) will be favoured at
Low pressure and low temperature
high temperature and low pressure
high pressure and low temperature
high pressure and low temperature
For the reaction N2 (g) + O2 (g) ⇌ 2NO (g), the equilibrium constant is K1. The equilibrium constant is K2 for the reaction 2NO (g) + O2(g) ⇌ 2NO2 (g). What is K for the reaction 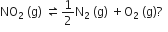
1/(4K1K2)
[1/K1K2]1/2
1/(K1K2)
1/(K1K2)
What is the pH of the resulting solution when equal volumes of 0.1 M NaOH and 0.01 M HCl are mixed?
12.65
2.0
7.0
7.0
Which one of the following pairs of the solution is not an acidic buffer?
HClO4 and NaClO4
CH3COOH and CH3COONa
H2CO3 and Na2PO4
H2CO3 and Na2PO4
