 Multiple Choice Questions
Multiple Choice QuestionsCH3COOH CH2BrCOOH
This reaction is called
Schotton-Bauman reaction
Finkelstin reaction
Hell-Volhard-'Zelinsky reaction
none of the above
Arrange the following in the decreasing order of their melting points:
I. Heptane II. Octane
III. Nonane IV. Decane
IV > II > III > I
IV > III > I > II
I > II > IV > III
II > III > I > IV
Which of the following is not a cumulated diene?
Hexa - 2 , 3 - diene
Penta - 1 , 3 - diene
Hexa - 1 , 2 - diene
Penta - 2 , 3 - diene
In the eclipsed conformation of ethane, the dihedral angle between the hydrogen atoms of adjacent methyl groups is :
60°
120°
0°
180°
The major product of the addition of water molecule to propyne in the presence of mercuric sulphate and dilute sulphuric acid is :
ethanal
2-propanol
propane
propanone
Which of the following statements is not an essential feature of an optically active molecule ?
It will rotate the plane of polarised light
It will have a non-superimposable mirror image
It will have no element of symmetry
It will have an asymmetric carbon atom
The reagent which could distinguish between 1-hexyne and 1-hexene is :
Ag
KMnO4
Br2 in CCl4
H2SO4
Choose the correct reagent required to carry out the transformation :
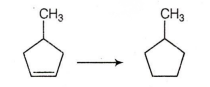
Zn + conc. HCl
conc. H2SO4
Li then H2O
H2/Pt
