 Multiple Choice Questions
Multiple Choice QuestionsA bimetallic strip consists of metals X and Y. It is mounted rigidly at the base as shown. The metal X has a higher coefficient of expansion compared to that for metal Y. When the bimetallic strip is placed in a cold bath
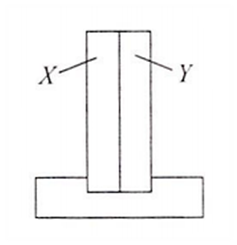
it will bend towards the right
it will bend towards the left
it will not bend but shrink
it will neither bend or shrink
Assertion: In an isolated system the entropy increases.
Reason: The processes in an isolated system are adiabatic.
If both assertion and reason are true and reason is the correct explanation of the assertion
If both assertion and reason are true but reason is not the correct explanation of the assertion
If assertion is true, but reason is false
Both assertion and reason are false statements
Assertion: The Carnot cycle is useful in understanding the performance of heat engines.
Reason: The Carnot cycle provides a way of determining the maximum possible efficiency achievable with reservoirs of given temperatures.
If both assertion and reason are true and reason is the correct explanation of the assertion
If both assertion and reason are true but reason is not the correct explanation of the assertion
If assertion is true, but reason is false
Both assertion and reason are false statements
Assertion: When a glass of hot milk is placed in a room and allowed to cool, its entropy decreases.
Reason: Allowing hot object to cool does not violate the second law of thermodynamics.
If both assertion and reason are true and reason is the correct explanation of the assertion
If both assertion and reason are true but reason is not the correct explanation of the assertion
If assertion is true, but reason is false
Both assertion and reason are false statement
Assertion: Reversible systems are difficult to find in real world.
Reason : Most processes are dissipative in nature.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
Assertion: Air quickly leaking out of a balloon becomes cooler
Reason: The leaking air undergoes adiabatic expansion
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
Assertion: In pressure-temperature (P-T) phase diagram of water, the slope of the melting curve is found to be negative.
Reason: Ice contracts on melting to water
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
N moles of a monoatomic gas is carried round the reversible rectangular cycle ABCDA as shown in the diagram. The temperature at A is To. The thermodynamic efficiency of the cycle is
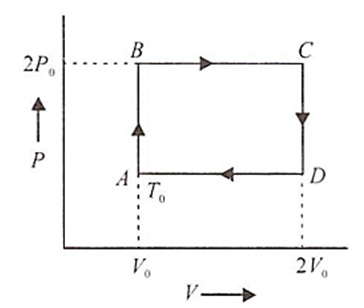
15 %
50 %
20 %
25 %
Assertion: Thermodynamic process in nature are irreversible.
Reason: Dissipative effects cannot be eliminated.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
Assertion: When a bottle of cold carbonated drink is opened, a slight fog forms around the opening.
Reason: Adiabatic expansion of the gas causes lowering of temperature and condensation of water vapours.
if both assertion and reason are true and the reason is the correct explanation of the assertion.
if both assertion and reason are true and the reason is not the correct explanation of the assertion.
if assertion is true but reason is false
if both assertion and reason are false statements
