 Multiple Choice Questions
Multiple Choice QuestionsAssertion: Anilinium chloride is more acidic than ammonium chloride.
Reason : Anilinium ion is resonance-stabilised.
If both assertion and reason are true and reason is the correct explanation of the assertion.
If both assertion and reason are true but reason is not the correct explanation of the assertion.
If assertion is true, but reason is false.
Both assertion and reason are false statements.
In the reaction :
C6H5CHO + C6H5NH2 C6H5N=HCC6H5 + H2O, the compound C6H5N=HCC6H5 is known as :
aldol
Schiffs base
Schiff's reagent
Benedict's reagent
IUPAC name of CH3-CH(CH2CH3)-CH2-CH(CN)-CH3 is :
2-cyano-3-methylhexane
2.4-dimethylhexanenitrile
3-methyl-5-cyanohexane
2-cyano-3-methylhexane
Aromatic nitriles (ArCN) are not prepared by reaction:
ArX + KCN
ArN2++ CuCN
ArCONH2+ P2O5
ArCONH2+ SOCl2
Melting points are normally the highest for
tertiary amides
secondary amides
primary amides
amines.
Assertion: Boiling and melting points of amides are higher than corresponding acids.
Reason: It is due to strong intermolecular hydrogen bonding in their molecules.
If both assertion and reason are true and the reason is a correct explanation of the assertion.
If both the assertion and reason are true but the reason is not a correct explanation of the assertion.
If the assertion is true but the reason is false.
If both the assertion and reason are false.
The basicity of aniline is weaker in comparison to that of methylamine due to:
hyper conjugative effect of Me-group in MeNH2
resonance effect of the phenyl group in aniline
the lower molecular weight of methylamine as compared to that of aniline
resonance effect of -NH2 group in MeNH2
An amine C3H9N reacts with benzene sulphonyl chloride to form a white precipitate which is insoluble in aq. NaOH. The amine is
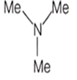
![]()
![]()
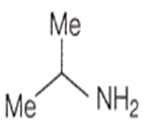
The reaction of aniline with chloroform under alkaline conditions leads to the formation of
phenylcyanide
phenylisonitrile
phenylcyanate
phenylisocyanate
