 Multiple Choice Questions
Multiple Choice QuestionsAn excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetraaquachromium (III) chloride. The number of moles of AgCl precipitate would be
0.001
0.002
0.003
0.003
Which of the following acids does not exhibit optical isomerism?
Maleic acid
α-amino acids
Lactic acid
Lactic acid
Which one of the following is an outer orbital complex and exhibits paramagnetic behaviour?
[Ni(NH3)6]2+
[Zn(NH3)6]2+
[Cr(NH3)6]3+
[Cr(NH3)6]3+
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni (II).Which of the following statements is not true?
Red Complex has a square planar geometry
Complex has symmetrical H- bonding
Red complex has a tetrahedral geometry
Red complex has a tetrahedral geometry
Low spin complex of d6 -cation in an octahedral field will have the following energy
(Δo = Crystal field splitting energy in an octahedral field,
P= electron pairing energy)
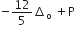

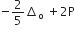
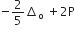
The complexes [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6] are the examples of which type of which type of isomerism?
Ionisation isomerism
Coordination isomerism
Geometrical isomerism
Geometrical isomerism
The d- electron configurations of Cr3+, Mn2+ , Fe2+, and Co2+ are d4 ,d5, d6 and d7 respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
(At. no. Cr = 24, Mn = 25, Fe =26, Co = 27)
[Fe(H2O)6]2+
[Co(H2O)6]2+
[Cr(H2O)6]2+
[Cr(H2O)6]2+
Of the following complex ions, which is diamagnetic in nature?
[Ni(CN)4]2-
[CuCl4]2-
[CoF6]3-
[CoF6]3-
The sum of coordination number and oxidation number of the metal M in the complex [M(en)2(C2O4)]Cl (where en is ethylenediamine) is
9
6
7
7
