 Multiple Choice Questions
Multiple Choice QuestionsStandard electrode potential of three metal X, Y and Z are -1.2 V, +0.5 V and -3.0 V respectively. The reducing power of these metals will be
Y > X > Z
Z> X> Y
X > Y > Z
X > Y > Z
If the Eocell for a given reaction has a negative value then which of the following gives the correct relationships for the values of ΔGo and Keq ?
ΔGo < 0; Keq > 1
ΔGo < 0; Keq < 1
ΔGo > 0; Keq < 1
ΔGo > 0; Keq < 1
The electrode potentials for
Cu2+ (aq) + e- → Cu+ (aq) and Cu+ (aq) + e- →Cu (s)
are +0.15 V and +0.50V respectively. The value of  will be
will be
0.325 V
0.650 V
0.150 V
0.150 V
Standard electrode potential for Sn4+ / Sn2+ couple is +0.15 V and that for the Cr3+/Cr couple is -0.74. These two couples in their standard state are connected to make a cell. The cell potential will be
+0.89 V
+0.18 V
+1.83 V
+1.83 V
A solution contains Fe2+ Fe3+, and I- ions. This solution was treated with iodine at 35o C.Eo for Fe3+/ Fe2+ is +0.77 V and Eo for I2/ 2 I- = 0.536 V. The favourable redox reaction is
I2 will be reduced to I-
There will be No redox reaction
I- will be oxidised to I2
I- will be oxidised to I2
In qualitative analysis, the metals of group I can be separted from other ions by precipitating them as chloride salts. A solution initially contains Ag+ and Pb2+ at a concentration of 0.10 M. Aqueous HCl is added to this solution until the Cl- concentration is 0.10 M. What will be the concentration of Ag+ and Pb2+ be at equilibrium? (Ksp for AgCl = 1.8 x 10-10, Ksp for PbCl2 = 1.7 x 10-5)
[Ag+] = 1.8 x 10-7 M; [Pb2+] = 1.7 x 10-6 M
[Ag+] = 1.8 x 10-11 M; [Pb2+] = 8.5 x 10-5 M
[Ag+] = 1.8 x 10-9 M; [Pb2+] = 1.7 x 10-3 M
[Ag+] = 1.8 x 10-9 M; [Pb2+] = 1.7 x 10-3 M
For the reaction of silver ions with copper metal, the standard cell potential was found to be +0.46 V at 25o C. The value of standard Gibbs energy, ΔGo will be (F = 96500 C mol-1 )
-89.0 kJ
-89.0 J
-44.5 kJ
-44.5 kJ
An increase in equivalent conductance of strong electrolyte with dilution is mainly due to
the increase in ionic mobility of ions
100% ionisation of electrolyte at normal dilution
the increase in both, ie, the number of ions and ionic mobility of ions
the increase in both, ie, the number of ions and ionic mobility of ions
Which of the following expression correctly represents the equivalent conductance at infinite dilution of Al2(SO4)3. Given that  are the equivalent conductance at infinite dilution of the respective ions.
are the equivalent conductance at infinite dilution of the respective ions.
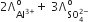
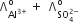


Consider the following relations for emf of an electrochemical cell
A) Emf of cell = (oxidation potential of anode) - (reduction potential of cathode)
B) Emf of cell = (oxidation potential of anode) - ( reducation potential of cathode)
C) Emf of cell = (oxidation potential of anode)- (reduction potential of cathode)
D) Emf of cell = (oxidation potential of anode) - (oxidation potential of anode)-(oxidation potential of cathode)
(C) and (A)
(A) and (B)
(C) and (D)
(C) and (D)
