 Multiple Choice Questions
Multiple Choice QuestionsIn a mixture, two enantiomers are found to be present in 85% and 15% respectively. The enantiomeric excess (ee) is
85%
15%
70%
60%
The IUPAC name of the compound having the formula CCl3CH2CHO is
2, 2, 2-trichloropropanal
1,1, 1-trichloropropanal
3, 3, 3-trichloropropanal
1, 2, 1-dichloromethanal
Best reagent for nuclear iodination of aromatic compounds is
KI/ CH3COCH3
I2/CH3CN
KI/ CH3COOH
I2 / HNO3
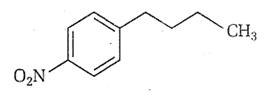
Identify the correct method for the synthesis of the compound shown below from the following alternatives.
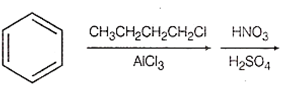
![]()
![]()
![]()
Which one of the following methods are used to prepare Me3COEt with good yield?
Mixing EtONa with Me2CCl
Mixing Me2CONa with EtCl
Heating a mixture of (1:1) EtOH and Me2COH in the presence of conc. H2SO4
Treatment of Me3COH with EtMgI
Under identical conditions, the SN1 reaction will occur most efficiently with:
tert-butyl chloride
1-chlorobutane
2-methyl-1-chloropropane
2-chlorobutane
Identify the method by which Me3CCO2H can be prepared:
Treating 1 mole of MeCOMe with 2 moles of MeMgl
Treating 1 mole of MeCO3Me with 3 moles of MeMgI
Treating 1 mole of MeCHO with 3 moles of MeMgl
Treating 1 mole of dry ice with 1 mol of MeCMgI
The correct statement regarding the following compounds is
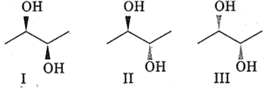
all three compounds are chiral
only I and II are chiral
I and III are diastereomers
only I and III are chiral
The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is:
3° < 2° < 1°
3° > 2° > 1 °
3° < 2° > 1°
3° > 2° < 1 °
