 Multiple Choice Questions
Multiple Choice QuestionsThe compound C7H8 undergoes the following reactions:
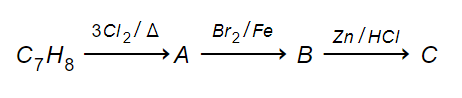
The product 'C' is
m-bromotoluene
o-bromotoluene
p-bromotoluene
3-bromo-2,4,6-trichlorotoluene
Hydrocarbon (A) reacts with bromine by substitution to form an alkyl bromide which by Wurtz reaction is converted to a gaseous hydrocarbon containing less than four carbon atoms. (A) is
CH ≡ CH
H2C = CH2
CH4
CH3-CH3
1-butyne on oxidation with hot alkaline KMnO4 would yield. Which of the following as end product?
CH3CH2CH2COOH
CH3CH2COOH
CH3CH2CH2COOH + CO2 + H2O
CH3CH2CH2COOH + HCOOH
A cubic unit cell of a metal with a molar mass of 63.55 g mol-1 has an edge length of 362 pm. Its density is 8.92 g cm-3. The type of unit cell is
Primitive
Face centred
End centred
Body Centred
Which of the following is the major product in the reaction of HOBr with propene?
2-bromo, 1-propanol
3-bromo,1-propanol
2-bromo, 2-propanol
1-bromo, 2-propanol
Which of the following is the correct IUPAC name?
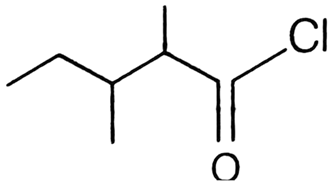
3,4 -dimethyl pentanoyl chloride
1-chloro-1-oxo-2, 3-dimethyl pentane
2-ethyl-3-methyl butanoyl chloride
2,3-dimethyl pentanoyl chloride
The pair of boiling point and compound are given as,
C6H6 (80C); CH3OH (65C); C6H5NO2 (212C) and C6H5NH2 (184C)
Which will show lowest vapour pressure at room temperature?
C6H6
CH3OH
C6H5NO2
C6H5NH2
In the following conversion,

Identify 'A' from the following option
NaBD4
LiAID4
Mg, ether/D2O
BH3, D2O
Ethers like ROR can be cleaved by concentrated HI but not by HCl because
I- is a weaker nucleophile than Cl-
I- is stronger nucleophile than Cl-
SN1 mechanism carried out in this reaction is rapidly in presence of HI.
None of the above
