 Multiple Choice Questions
Multiple Choice QuestionsThe size of colloidal particle is :
10-3 to 109 m
10-9 to 10-12 m
10-6 to 10-9 m
10-12 to 10-19 m
Assertion : Aqueous gold colloidal solution is red in colour.
Reason : The colour arises due to scattering of light by colloidal gold articles.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
A packet of colloidal system is taken in which colloidal particles are still. Two electrodes are taken in system and voltage is applied so that liquid medium moves under the influence of electric field. This phenomenon is called
electrodialysis
electroosmosis
electrophoresis
None of these.
Assertion: Physical absorption of molecules takes place on the surface only.
Reason: In this process, the bonds of the absorbed molecules are broken.
If both assertion and reason are true and reason is a correct explanation of the assertion.
If both the assertion and reason are true but the reason is not a correct explanation of the assertion.
If the assertion is true but the reason is false.
If both the assertion and reason are false.
The correct statement regarding the following energy diagrams is
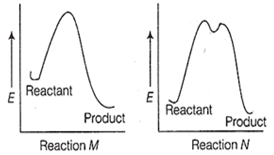
Reaction M is faster and less exothermic than reaction N
Reaction M is slower and less exothermic than reaction N
Reaction M is faster and more exothermic than reaction N
Reaction M is slower and more exothermic than reaction N
The amount of electrolytes required to coagulate a given amount of AgI colloidal solution (-ve charge) will be in the order
NaNO3 > Al2(NO3)3 > Ba(NO3)2
Al2(NO3)3 > Ba(NO3)2 > NaNO3
Al2(NO3)2 > NaNO3 > Ba(NO3)2
NaNO3 > Ba(NO3)2 > Al2(NO3)3
The decomposition of hydrogen peroxide can be slowed by the addition of acetamide. The latter acts as a
detainer
stopper
promoter
inhibitor
The formation of colloid from suspension is
peptisation
condensation
fragmentation
sedimentation
