CBSE Multiple Choice Questions
Multiple Choice QuestionsOne mole of an ideal monoatomic gas is heated at a constant pressure from 0°C to 100°C. Then the change in the internal energy of the gas is (Given, R = 8.32 J mol-1 K-1)
0.83 × 103 J
4.6 × 103 J
2.08 × 103 J
1.25 × 103 J
The rms speed of oxygen is v at a particular temperature. If the temperature is doubled and oxygen molecules dissociate into oxygen atoms, the rms speed becomes
v
2 v
4 v
Determine the mean free path of argon molecules under normal condition knowing that the molecule diameter is 0.4 nm.
48 nm
60 nm
52 nm
71 nm
At what temperature will the rms speed of air molecules be double that NTP ?
519° C
619° C
719° C
819° C
Six molecules speeds 2 unit, 5 unit, 3 unit, 6 units, 3 unit and 5 unit respectively. The rms speed is
4 unit
1.7 unit
4.2 unit
5 unit
The equation of state for n moles of an ideal gas is pV = nRT, where R is a constant. The SI unit for R is
Jk-1 per molecule
JK-1 mol-1
JKg-1K-1
Jk-1g-1
Volume versus temperature graphs for a given mass of an ideal gas are shown in figure. At two different values of constant pressure, what can be inferred about relation between p1 and p2 ?
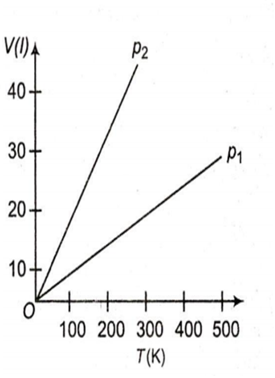
p1 > p2
p1 = p2
p1 < p2
data is insufficient
For molecules of a gas have speeds 1, 2, 3 and 4 km/s. The value of the root mean square speed of the gas molecules is
2.5 km/s

