CBSE Multiple Choice Questions
Multiple Choice QuestionsThe amount of heat energy required raising the temperature of 1g of helium at NTP, from T1K and T2K is
3/8 NaKB(T2-T1)
3/2 NaKB(T2-T1)
3/4 NaKB(T2-T1)
3/4 NaKB(T2-T1)
The piece of iron is heated in a flame. If first becomes dull red then becomes reddish yellow and finally turns to white hot. The correct explanation for the above observation is possible by using,
Stefan's law
Wien's displacement law
Kirchoff's law
Kirchoff's law
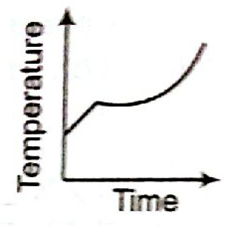
Liquid oxygen at 50 K is heated to 3000 K at a constant pressure of 1 atm. The rate of heating is constant. which one of the following graphs represents the variation of temperature with time?



The charge following through a resistance R varies with time t as Q = at - bt2, where a and b are positive constants. The total heat produced in R is,
a3R/3b
a3R/2b
a3R/b
a3R/b
A slab of stone of area of 0.36 m2 and thickness 0.1 m is exposed on the lower surface to steam at 100oC. A block of ice at 0o C rests on the upper surface of the slab. In one hour 4.8 kg of ice is melted. The thermal conductivity of slab is
(Given latent heat of fusion of ice = 3.36 x 105 J Kg-1)
1.24 J/m/s/oC
1.29 J/m/soC
2.05 J/m/so C
2.05 J/m/so C
One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure.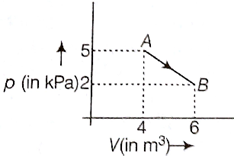
The change in internal energy of the gas during the transition is
20 kJ
-20 kJ
20 J
20 J
An ideal gas goes from state A to state B via three different processes as indicated in the p-V diagram.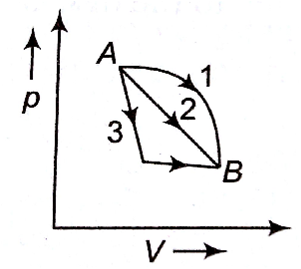
If Q1, Q2, Q3 indicate the heat absorbed by the gas along the three processes and ΔU1, ΔU2, ΔU3 indicate the change in internal energy along the three processes respectively, then
Q1> Q2> Q3 and ΔU1=ΔU2= ΔU3
Q3> Q2> Q1 and ΔU1=ΔU2= ΔU3
Q1= Q2= Q3 and ΔU1=ΔU2= ΔU3
Q1= Q2= Q3 and ΔU1=ΔU2= ΔU3
The two ends of a metal rod are maintained at temperatures 100o C and 110o C. The rate of heat flow in the rod is found to be 4.0 J/s. If the ends are maintained at temperatures 200o C and 210o C, the rate of heat flow will be
44.0 J/s
16.8 J/s
8.0 J/s
8.0 J/s
If the radius of a star is R and it acts as a black body, what would be the temperature of the star, in which the rate of energy production is Q?
Q/4πR2σ
(Q/4πR2σ)-1/2
(4πR2Q/σ)1/4
(4πR2Q/σ)1/4
Coefficient of linear expansion of brass and steel rods are 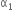 and
and  . Lengths of brass and steel rods are l1 and l2 respectively. If (I2 - I1) is maintained same at all temperatures, which one of the following relations holds good?
. Lengths of brass and steel rods are l1 and l2 respectively. If (I2 - I1) is maintained same at all temperatures, which one of the following relations holds good?





