CBSE Multiple Choice Questions
Multiple Choice QuestionsAn ideal gas is compressed isothermally until its pressure is doubled and then allowed to expand adiabatically to regain its original volume (γ = 1.4 and 2-1.4 = 0.38). The ratio of the final to initial pressure is
0.76 : 1
1 : 1
0.66 : 1
0.86 : 1
The molar specific heats of an ideal gas at constant pressure and volume are denoted by CP and CV respectively. If and R is the universal gas constant, then CV is equal to
γR
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio of for the gas is
2
A scientist proposes a new temperature scale in which the ice point is 25 X (X is the new unit of temperature) and the steam point is 305 X. The specific heat capacity of water in this new scale is (in J kg-1X-1)
4.2 × 103
3.0 × 103
1.2 × 103
1.5 × 103
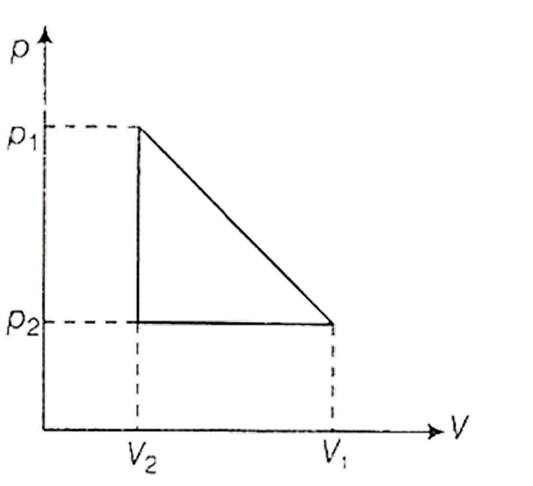
One mole of a van der Waals' gas obeying the equation
undergoes the quasi-static cyclic process which is shown in the p-V diagram. The net heat absorbed by the gas in this process is
A heating element of resistance r is fitted inside an adiabatic cylinder which carries a frictionless piston of mass m and cross-section A as shown in diagram. The cylinder contains one mole of an ideal diatomic gas. The piston current flows through the element such that the temperatures rises with time t as ΔT = αt + βt2 ? (α and β are constants), while pressure remains constant. The atmospheric pressure above the piston is P0. Then
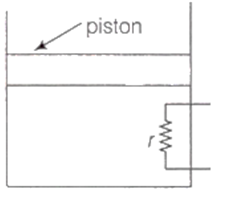
he rate of increase in internal energy is
the current flowing in the element is
the piston moves upwards with constant acceleration
the piston moves upwards with constant speed
The pressure p, volume V and temperature T for a certam gas are related by , where A and B are constants. The work done by the gas when the temperature changes from T1 to T2 while the pressure remains constant, is given by
A (T2 − T1) + B (T22 − T12)
Assertion: In an adiabatic process, change in internal energy of a gas is equal to work done on or by the gas in the process.
Reason: Temperature of gas remains constant in an adiabatic process.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.

