CBSE
Class 10 Class 12
Download this Science Pre Board 1 for taking the test offline or sharing with your friends. Once you are done with all the answers to the questions, Go ahead with answer key to check your answers.

General Instructions:

| 1. | Why should a magnesium ribbon be cleaned before burning in air? | [1] |
| 2. | What are the raw materials for photosynthesis? | [1] |
| 3. | An element M is in the third group of the periodic table. Write the formulae of its chloride and oxide. | [2] |
| 4. | A convex mirror used for rear-view on an automobile has a radius of curvature of 3.00 m. If a bus is located at 5.00 m from this mirror, find the position, nature and size of the image. | [2] |
| 5. |
Why is Government of India imposing a ban on the use of polythene bags? Suggest two alternatives to these bags and explain how this ban is likely to improve the environment. | [2] |
| 6. | What are artificial magnets? What are their common shapes? | [3] |
| 7. | Draw a schematic circuit diagram consisting a battery, a plug key, an ammeter and a bulb all connected in series with a voltmeter connected in parallel with the bulb. | [3] |
| 8. |
A cross was made between pure breeding pea plants one with round and green seeds and the other with wrinkled and yellow seeds. (a) Write the phenotype of F1 progeny. Give reason for your answer. (b) Write the different types of F2 progeny obtained along with their ration when F1 progeny was selfed. | [3] |
| 9. |
Given below are some elements of the modern periodic table: 4Be, 9Fe, 14Si, 19K, 20Ca (i) Select the element that has one electron in the outermost shell and write its electronic configuration. (ii) Select two elements that belong to the same group. Give reason for your answer. (iii) Select two elements that belong to the same period. Which one of the two has bigger atomic size? | [3] |
| 10. |
Write the functions of the following in human female reproductive system: Ovary, oviduct, uterus. How does the embryo get nourishment inside the mother's body? Explain in brief. | [3] |
List and explain in brief three methods of contraception.
| 11. |
Explain Mendel’s law of independent inheritance. Give one example. | [3] |
| 12. | A concave mirror of focal length 15 cm can form a magnified, erect as well as inverted image of an object placed in front of it. Justify this statement stating the position of the object with respect to the pole of the mirror in both the cases for obtaining the images. | [3] |
| 13. | When hydrochloric acid is added to marble pieces, a gas (A) is evolved. On passing gas A through lime water, a white precipitate of (B) is formed. When excess of A is passed, B dissolves due to the formation of soluble C. Identify A, B and C. Explain the reactions. | [3] |
| 14. |
Define evolution. How does it occur? Describe how fossils provide us evidences in support of evolution. | [3] |
| 15. |
List four points of significance of reproductive health in a society. Name any two areas related to the reproductive health which have improved over the past 50 years in our country. | [3] |
| 16. | Draw the pattern of magnetic field lines around a straight conductor carrying electric current. Apply right hand thumb rule to mark the direction of magnetic field. | [5] |
| 17. |
(a) Define the term 'isomers'. (c) Give the electron dot structures of the above two compounds. | [5] |
An organic compound A has the molecular formula C2H6O. On oxidation in air in presence of heated copper as catalyst, it is oxidised to CH3COOH. What is the compound ‘A?
Give the equation for the reaction.
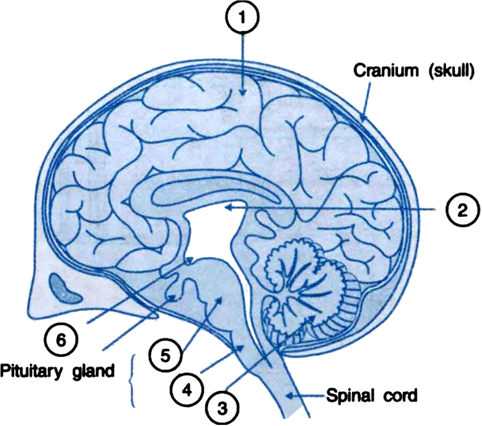
| 19. |
(a) A person cannot read newspaper placed nearer than 50 cm from his eyes. Name the defect of vision he is suffering from. Draw a ray diagram to illustrate this defect. List its two possible causes. Draw a ray diagram to show how this defect may be corrected using a lens of appropriate focal length. | [5] |
A certain metal ‘X’ lies between potassium and magnesium in the activity series.
Answer the following questions:
(a) What will happen when is added to cold water?
(b) What reaction will take place between X and dil. hydrochloric acid?
What is sustainable management? Why is reuse considered better in comparison to recycle ?
Management of forest and wild life resources is a very challenging task. Why ? Give any two reasons.
Study the picture given below and comment on the encircled organisms with respect to
(i) the category according to the food they eat.
(ii) trophic level to which they belong.
(iii) percentage of energy available at their trophic level.
(iv) two abiotic components of the ecosystem inhabited by them.
(v) energy used for food production by the producers.

| 22. |
A gas is liberated immediately with a brisk effervescence when you add acetic acid to sodium hydrogen carbonate powder in a test tube. Name the gas and describe the test that confirms the identity of the gas. | [2] |
| 23. |
Dry raisins were soaked in water for 2 hours, to determine the percentage of water absorbed by raisins. Before final weighing of swollen raisins, the extra water left on the surface of soaked raisins was removed by: | [2] |
gently rubbing with cotton cloth
hot air blower
dry cotton wool
dry cotton wool
| 24. |
In the figure, the parts marked A, B and C is sequentially: | [2] |
Plumule, Radicle and Cotyledon
Radicle, Plumule and Cotyledon
Plumule, Cotyledon and Radicle
Plumule, Cotyledon and Radicle
| 25. |
Iron nails were dipped in an aqueous solution of copper sulphate. after about 30 mintues, it was observed that the colour of the solution changed from | [2] |
Colourless to light green
Blue to light green
Blue to colurless
Blue to colurless
| 26. |
A student has obtained a magnified image of a flame on a screen using a convex lens. To draw the corresponding ray diagram to show the image formation, which of the following two rays whose paths after refraction are shown, should he select?
| [2] |
I and II
II and III
III and IV
III and IV
| 27. | Find the effective resistance in the given circuit?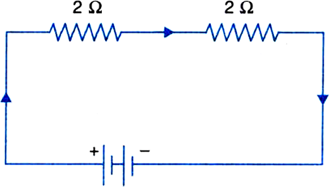 | [2] |