CBSE
Class 10 Class 12
Download this Science Pre Board 3 for taking the test offline or sharing with your friends. Once you are done with all the answers to the questions, Go ahead with answer key to check your answers.

General Instructions:

| 1. | What is a reflex action? | [1] |
| 2. | Name the methods of asexual reproduction. | [1] |
| 3. | Write the group member with atomic number for each of the following elements: (i) F (at. no. 9) (ii) K (at. no. 19) | [2] |
| 4. | Which type of phenomenon (reflection or refraction) occurs when light falls on (i) a highly polished surface like a mirror and (ii) a transparent medium like glass or water? | [2] |
| 5. | Explain, why are fossil fuels classified a non-renewable sources of energy? | [2] |
| 6. | Balance the following chemical equations: | [3] |
| 7. | When hydrochloric acid is added to marble pieces, a gas (A) is evolved. On passing gas A through lime water, a white precipitate of (B) is formed. When excess of A is passed, B dissolves due to the formation of soluble C. Identify A, B and C. Explain the reactions. | [3] |
| 8. | How is the problem of dobling of the amount of DNA solved ? | [3] |
| 9. | What is the full form of ATP? How is it form? How much energy it releases when broken down? What are its uses? | [3] |
| 10. | The following table shows the position of six elements A, B, C, D, E and 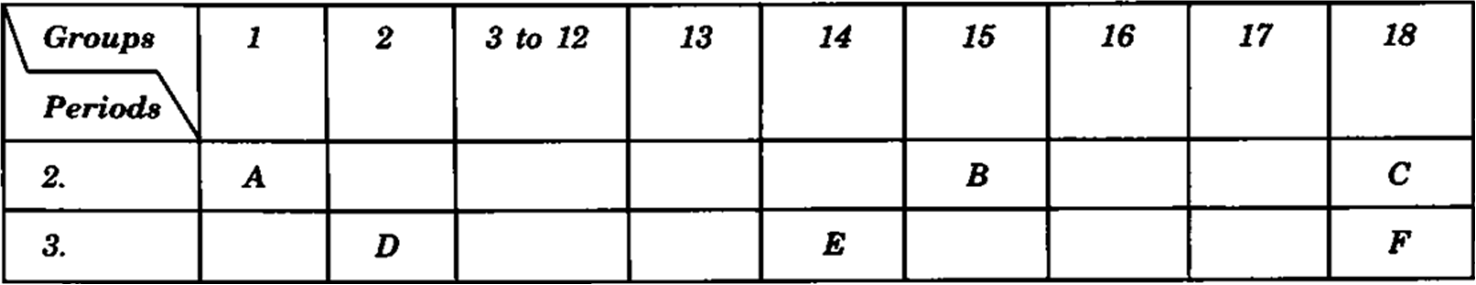 Using the above table answer the following questions: (a) Which element will form only covalent compounds? | [3] |
| 11. |
The sex of a newborn child is a matter of chance and none of the parents may be considered responsible for it. Justify this statement with the help of flow chart showing determination of sex of a newborn. | [3] |
| 12. | By drawing ray diagrams, explain the formation of image when an object is placed on the principal axis of a convex lens at the following positions: (i) At infinity (ii) Beyond 2F1 (iii) At 2F1 (iv) Between F1 and 2F1 (v) At focus F1 (vi) Between focus F1 and optical centre O. | [3] |
| 13. | An electric lamp, whose resistance is 20 Ω, and a conductor of 4 Ω resistance are connected to a 6 V battery. Calculate (a) the total resistance of the circuit, (b) the current through the circuit, and (c) the potential difference across the electric lamp and conductor. Fig. An electric lamp connected in series with a resistor of 4 Ω to a 6 V battery | [3] |
| 14. | Define genetics. What is the contribution of Mendel in this branch of Biology? | [3] |
| 15. | The flow of current in a circular loop of wire creates a magnetic field at the centre. How may the existence of this field may be detected? State the rule which helps to predict the direction of the magnet field? 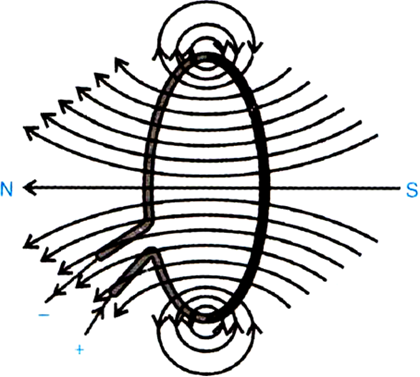 | [3] |
| 16. | What happens when: (i) Lead is heated to 400–500°C in air. | [5] |
| 17. |
(a) In a tabular form, differentiate between ethanol and ethanoic acid under the following heads: (i) Physical state (ii) Taste (iii) NaHCO3 test (iv) Ester test (b) Write a chemical reaction to show the dehydration of ethanol. | [5] |
Classify the following compounds as alkane, alkene or alkyne.
C3H4, C2,H6 C2H4, C6H14, C2H2, C4H8, C5H12’ C5H8, C3H8
Explain, how did you arrive at your conclusion in each case.
| 18. | List the major endocrine glands found in human body. Write their functions. | [5] |
| 19. | Discuss the refraction of light through a prism. What are angle of deviation and angle of emergence? How are these angles related to the angle of incidence and the angle of prism? | [5] |
| 20. | Draw a schematic diagram of household/domestic wiring. Give its essential features. | [5] |
What was Chipko Andolan? How did the Andolan ultimately benefit the local people and the environment?

| 22. |
When you add about 2 ml of acetic acid to a test tube containing an equal amount of distilled water and leave the test tube to settle after shaking its contents, what will you observe in the test tube after about 5 minutes? | [2] |
A white precipitate settling at its bottom
A clear colorless solution
A layer of water over the layer of acetic acid.
A layer of water over the layer of acetic acid.
| 23. |
A student takes 2 mL acetic acid in a dry test tube and adds a pinch of sodium hydrogen carbonate to it. He makes the following observations: I. A colorless and odorless gas evolves with a brisk effervescence. II. The gas turns lime water milky when passed through it. III. The gas burns with an explosion when a burning splinter is brought near it. IV. The gas extinguishes the burning splinter that is brought near it. The correct observations are: | [2] |
I, II, and III
II, III and IV
III, IV and I
III, IV and I
| 24. |
During the course of an experiment, 'to determine the percentage of water absorbed by raisins', raisins are weighed | [2] |
every half an hour.
every hour.
once - only after completing the experiment.
once - only after completing the experiment.
| 25. |
Study the following diagrams showing various stages of binary fission in Amoeba: The correct sequence of these diagrams should be:
| [2] |
I, IV, III,II, V
I, III, IV, II, V
I, II, IV, III, V
I, II, IV, III, V
| 26. |
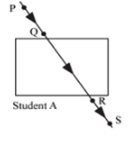
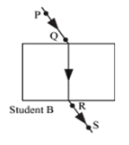
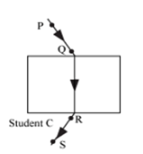
Four students A, B, C and D traced the paths of incident ray and the emergent ray by fixing pins P and Q for incident ray and pins R and S for emergent ray for a ray of light passing through a glass slab. The correct emergent ray was traced by the student: | [2] |

| 27. | Of the following groups which one contains good conductors of electricity | [2] |