CBSE
Class 10 Class 12
Download this Science Pre Board 4 for taking the test offline or sharing with your friends. Once you are done with all the answers to the questions, Go ahead with answer key to check your answers.

General Instructions:

| 1. | What are the three major regions of human brain? | [1] |
| 2. | Is an offspring exactly similar to its parent? Why or why not? | [1] |
| 3. | An element M is in the third group of the periodic table. Write the formulae of its chloride and oxide. | [2] |
| 4. | State the important properties of images formed by plane mirrors. | [2] |
| 5. | Explain how biomass is used as a fuel? | [2] |
| 6. | What happens when silver chloride is placed in sunlight in a china dish. Write the balanced chemical equation. | [3] |
| 7. | How are metal carbonates formed? Give an example along with the chemical equation of the reaction involved. | [3] |
| 8. | What is HIV-AIDS? How is it spread? | [3] |
| 9. | Explain the structure of stomata and function of guard cells. | [3] |
| 10. |
How many groups and periods are there in the modern periodic table? How do the atomic size and metallic character of elements vary as we move: (a) Down a group and (b) From left to right in a period | [3] |
| 11. |
Tabulate two distinguishing features between acquired traits and inherited traits with one example of each. | [3] |
| 12. | An object of size 5 cm is placed at a distance of 25 cm from the pole of a concave mirror of radius of curvature 30 cm. Calculate the distance and size of the image so formed. What will be the nature of the image? | [3] |
| 13. | Three resistors of magnitudes 12 Ω, 15 Ω and 20 Ω are connected in (i) series and (ii) parallel. Calculate the combined resistance in each case. | [3] |
| 14. | Briefly explain Stanley L. Miller and Harold C. Urey's experiment. | [3] |
| 15. | An electron enters a uniform magnetic field at right angles to it as shown in the figure. In which direction will this electron move? State the principle applied by you in finding the direction of motion of the electron.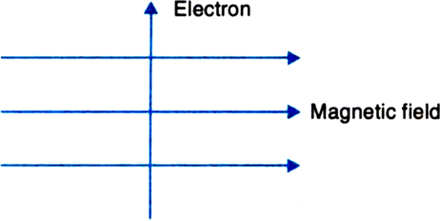 | [3] |
| 16. | (a) Show the formation of Na2O by the transfer of electrons between the combining atoms. | [5] |
| 17. |
(a) Define the term 'isomers'. (c) Give the electron dot structures of the above two compounds. | [5] |
Light a bunsen burner. When do you get
(i) yellow, sooty flame
(ii) blue flame
| 18. | Draw the structure of a neuron and explain its function. | [5] |
| 19. | A student finds the writing on the blackboard as blurred and unclear when sitting on the last desk in the classroom. He however, sees it clearly when sitting on the front desk at an approximate distance of 2 m from the blackboard. Draw ray diagrams to illustrate the formation of image of the blackboard writing by his eye-lens when he is seated at the (i) last desk (ii) front desk. Name the kind of lens that would help him to see clearly even when he is seated at the last desk. Draw a ray diagram to illustrate how this lens helps him to see clearly. | [5] |
Is it possible that the food chains have more than six steps? Support your answer with reasons.
Why are forest considered biodiversity hot spots? List two ways in which an individual can contribute effectively to the management of forests and wildlife.

| 22. |
The color of an aqueous solution of zinc sulphate as observed in the laboratory is: | [22] |
Green
Yellow
Blue
Blue

| 23. |
A student prepared 20% sodium hydroxide solution in a beaker containing water. The observations noted by him are given below. I. Sodium hydroxide is in the form of pellets. II. It dissolves in water readily. III. The beaker appears cold when touched from outside. IV. The red litmus paper turns blue when dipped into the solution. The correct observations are: | [2] |
I, II, and III
II, III and IV
III, IV and I
III, IV and I

| 24. |
Following are the steps involved in the experiment 'to determine the percentage of water absorbed by raisins'. They are not in proper sequence. I. Soak the raisins in fresh water. The correct sequence of steps is | [2] |
I, II, III, IV
II, I, IV, III
II, I, III, IV
II, I, III, IV
| 25. |
Identify the figures showing the process of budding in yeast. | [2] |
I, II AND III
II, III AND IV
I, II AND IV
I, II AND IV
| 26. |
Mohan obtained a sharp inverted image of a distant tree on the screen placed behind the lens. He then moved the screen and tried to look through the lens in the direction of the object. He would see: | [2] |
a blurred image on the wall of the laboratory.
an erect image of the tree on the lens.
no image as the screen has been removed
no image as the screen has been removed
| 27. | One ampere is equal to | [2] |