CBSE
Class 10 Class 12
Download this Chemistry Pre Board Paper 1 for taking the test offline or sharing with your friends. Once you are done with all the answers to the questions, Go ahead with answer key to check your answers.


| 1. |
‘Crystalline solids are anisotropic in nature’. What does this statement mean? | [1] |
| 2. |
Give one example each of 'oil in water' and 'water in oil' emulsion. | [1] |
| 3. |
Write the IUPAC name of | [1] |
| 4. |
Arrange the following compounds in increasing order of solubility in water: C6H5NH2, (C2H5)2NH, C2H5NH2 | [1] |
| 5. |
Name the base that is found in nucleotide of RNA only. | [1] |
| 6. |
Define an ideal solution and write one of its characteristics. | [2] |
| 7. |
Explain the mechanism of the following reaction: | [2] |
| 8. |
Define limiting molar conductivity. Why conductivity of an electrolyte solution decreases with the decrease in concentration? | [2] |
| 9. |
Name the two groups into which phenomenon of catalysis can be divided. Give an example of each group with the chemical equation involved. | [2] |
| 10. |
How will you convert Phenol to 2, 4, 6- trinitrophenol? | [2] |
| 11. |
Tungsten crystallizes in the body-centred cubic unit cell. If the edge of the unit cell is 316.5 pm, what is the radius of tungsten atom? | [3] |
| 12. |
Define each of the following terms: (i) Micelles (ii)Peptization (iii)Desorption | [3] |
| 13. |
The conductivity of 0.20 mol L-1 solution of KCl is 2.48 x 10-2 S cm-1. Calculate its molar conductivity and degree of dissociation (K+) = 73.56 S cm2 mol-1 and (Cl-)= 76.5 S (b) What type of battery is mercury cell? Why is it more advantageous than dry cell? | [3] |
| 14. |
Account for the following: (i) Acidic character increases from HF to HI. (ii) There is a large difference between the melting and boiling points of oxygen and sulphur. (iii) Nitrogen does not form pentahalide. | [3] |
| 15. |
A solution prepared by dissolving 8.95 mg of a gene fragment in 35.0 mL of water has an osmotic pressure of 0.335 torr at 25°C. | [3] |
| 16. |
Complete the following chemical equations: | [3] |
| 17. | Carbon monoxide is more effective agent that carbon below 983 K but above this temperature the reverse is true. How would you explain this? | [3] |
| 18. |
(a) Write the hybridization and shape of the following complexes: (i) [CoF6]3– (ii) [Ni (CN)4]2– (Atomic number: Co = 27, Ni = 28)
| [3] |
Write the name, stereochemistry and magnetic behavior of the following:
(At. nos. Mn = 25, Co = 27, Ni = 28)
(i) K4[Mn (CN)6]
(ii) [Co (NH3)5Cl]Cl2
(iii) K2[Ni (CN)4]
| 19. |
Although chlorine is an electron withdrawing group, yet it is ortho-, Para-directing in electrophilic aromatic substitution reactions. Explain why it is so? | [3] |
| 20. |
Give reasons for the following: | [3] |
| 21. |
(i) Write the product obtained when D-glucose reacts with H2N - OH. | [3] |
| 22. |
What are the following substances? Give one example of each one of them. (i) Tranquilizers (ii) Food preservatives (iii) Synthetic detergents | [3] |
| 23. |
After the ban on plastic bags, students of a school decided to make people aware of the harmful effects of plastic bags on the environment and Yamuna River. To make the awareness more impactful, they organised a rally by partnering with other schools and distributed paper bags to vegetable vendors, shopkeepers and departmental stores. All the students pledged not to use polythene bags in the future to save the Yamuna River. After reading the above passage, answer the following questions: <(i) What values are shown by the students? (ii) What are bio-degradable polymers? Give one example. (iii) Is polythene a condensation or an addition polymer ? | [4] |
| 24. | Starting with differential rate law equation for a first order reaction, derive the integrated rate law equation for a first order reaction. How is it related to the rate constant? | [5] |
| 25. |
(i) Which allotrope of phosphorus is more reactive and why? (ii) How the supersonic jet aeroplanes are responsible for the depletion of ozone layers? (iii) F2 has lower bond dissociation enthalpy than Cl2. Why? (iv) Which noble gas is used in filling balloons for meteorological observations? (v) Complete the equation: XeF2 + PF5 → | [5] |
(i) Name the elements of 3d transition series that show a maximum number of oxidation states. Why does this happen?
(ii) Which transition metal of 3d series has positive E0 (M2+/M) value and why?
(iii) Out of Cr3+ and Mn3+, which is a stronger oxidising agent and why?
(iv) Name a member of the lanthanoid series that is well-known to exhibit +2 oxidation state.
(v) Complete the following equation: MnO4- + 8H+ + 5e- -->
Write the products of the following reactions: 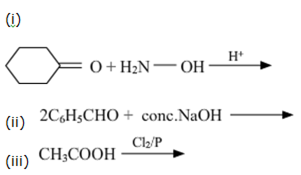
Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Benzaldehyde and Benzoic acid
(ii) Propanal and Propanone
Write the chemical equations to illustrate the following name reactions:
(i) Wolff-Kishner reduction
(ii) Aldol condensation
(iii) Cannizzaro reactionIllustrate the following reactions giving a suitable example for each.
(i) Cross aldol condensation
(ii) Decarboxylation